high-accuracy
advertisement

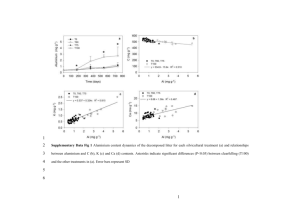
NAA Application at the INCT Halina Polkowska-Motrenko Institute of Nuclear Chemistry and Technology, Warsaw, Poland The Institute of Nuclear Chemistry and Technology (INCT) was established in 1983 after partition of the Institute of Nuclear Research in which it formerly existed as a Chemistry Division. The Institute has a leading role in Poland in implementing nuclear technologies, methods and instruments. The INCT is appointed as the main institution responsible for the development and implementation of nuclear analytical techniques in Poland. The Department of Analytical Chemistry of the INCT has been involved in the elaboration of INAA and RNAA methods for determination of selected elements in many different materials. INAA has been applied for multielemental analysis of rare and precious samples e.g. meteorites, concentrates and wastes from copper industry, forensic samples etc. and also for homogeneity testing of candidate CRMs. Elemental standardization is used. Contents of up to 23 elements can be quantified on the basis of determination of medium- and long-lived radionuclides. RNAA methods has been applied for determination of selected trace elements in different samples. Many radiochemical separation schemes enabling selective and quantitative separation of selected elements have been elaborated. Mainly ionexchange and extraction column chromatography but also liquid extraction and distillation have been applied. The INCT has developed a concept of the high-accuracy (definitive) RNAA methods on selected elements in biological matrices and such methods have been elaborated since 1986. The high-accuracy RNAA methods are applied in the certification process of CRMs for inorganic trace analysis as well as for the validation of other analytical procedures. High accuracy RNAA methods for selected elements in biological matrices • methods of guaranteed accuracy based on combination of: • neutron activation • very selective and quantitative postirradiation separation of indicator radionuclide by column chromatography • gamma-ray spectrometric measurements Basic steps in devising high-accuracy RNAA method Devising of mineralization method and separation scheme involving column chromatography Checking with the aid of radioactive tracers whether there is 100% recovery and high radiochemical purity of the analyte fraction No Yes NEUTRON IRRADIATION samples, standards, CRMs, blanks, neutron flux monitors Yes Radiochemical separation samples and one standard gamma-ray spectrometric measurements of samples and all standards Checking if all qualifying criteria have been met No No result is not accepted Ye s Result is accepted as obtained by the high-accuracy method The high-accuracy RNAA method is designed and elaborated according to rules assuring high accuracy: • All conditions should be optimized enabling selective isolation of an element with 100% yield confirmed by tracer experiments, • All potential sources of errors starting from sampling and sample dissolution up to gamma-spectrometric measurements should be identified at a stage of elaborating the method, and removed or appropriate corrective actions introduced into the procedure. • Whenever possible, the color of the ion in question (or its complex) added as a carrier should be used for visual control to safeguard against unexpected failure of the separation procedure • With each set of samples at least two standards should be irradiated, one of which is later on processed exactly as are the samples and the other is not. The specific activities of both standards should agree within predetermined limits; • Residual blank resulting from the contact of the sample with sample container should be measured in each series of determinations; In principle, the method should be a single element one while all parameters can be optimized with respect to this particular element. The method is equipped with some classification criteria providing protection against making gross errors. The criteria are as follows: • Visual control of the correctness of the separation procedure: in the retention stage on the chromatography columns the color band of analyte should not travel more than 1/3 of the bed length • Residual blank (may originating form the contact of the sample with packing material) is small (below a few percent of the analyte contents in analyzed sample) • Agreement of standards: the standard which has passed the whole analytical procedure should agree within predetermined limits (ca. few percent) with that measured directly • The result for at least one CRM irradiated and analyzed together with the samples should be in agreement with the certified value The result has to fulfil all of criteria to be accepted. 1000 100 200 300 400 500 Cd 527.7 keV 11 5 Cd 492.5 keV Cd - 11 5 115m In 336.6 keV 3000 11 5 Cd 261 keV 11 5 Cd 231 keV 11 5 Counts per channel 4000 2000 0 600 700 Energy, keV 800 900 1000 1100 1200 The limit of detection of the method has amounted to 0.5 ng g-1. The results obtained by this method are in excellent agreement with certified values for the CRMs spanning the Cd concentration range of more than 5 orders of magnitude. 3 10 y = 0.98805x + 0.02852 2 R = 0.99992 8 2 Experimental values mg g -1 10 1 10 7 0 10 6 5 -1 10 3 4 -2 10 2 1 -3 10 -3 10 -2 10 -1 10 0 10 Certified v alues mg g 1. 2. 3. 4. Ve rsieck ' s H uma n Ser um IAE A H -4 Anim al Muscle IN CT- TL -1 Te a Le av es IAEA H- 9 H um an Die t 5. 6. 7. 8. 1 10 2 10 -1 IN CT- MPH- 2 Mix ed Po lish He rbs CT A-O T L-1 Or ienta l T obacc o Le av es IAE A MA-M -2 Mussel T issue IAEA H- 8 H or se Kidne y Determina tion of Cd by the de finitive method in the ce rtified re fe re nce ma teria ls 3 10 The results are also precise as can be seen when the results are shown on the background of Horwitz and Thompson and Lowthian curves representing variation of reproducibility precision 140 120 Thompson and Lowthian curve RSD (%) 100 80 (Horwitz curve) x 1/2 60 40 Horwitz curve 20 0 1E-10 1E-9 1E-8 1E-7 1E-6 1E-5 -1 Mass fraction, (g g ) 1E-4 1E-3 This method can be also apply for Ni determination when the samples are irradiated with the high flux of fast neutrons by reaction 58Ni(n,p)58Co The limit of detection for Co amounted to 4x10-2 ng g-1 and 20 ng g-1 for Ni. High accuracy RNAA method for the determination of Mo in biological materials Nuclear reaction used for Mo determination 98Mo(n,γ)99Mo 99mTc may be subjected to interference originating from the nuclear fission reaction 235U(n,f)99Mo 99mTc The elaborated analytical scheme allows for the simultaneous determination of trace amount of Mo and U in biological materials. Uranium is determined by the reaction: 238U(n,γ)239U 239Np The detection limits amounted 0.15 ng g-1 for U and 2.5 ng g-1 for Mo. Uncertainty budget for Co determination in INCT-Tl-1 Tea Leaves source of uncertainty unit value Mass of sample mg 250 Mass of standard mg 20 Mass of neutron flux monitor mg 50 Residual blank ng 0.01 Neutron flux gradient 1.00±0.025 Neutron self-shielding/scattering Sample counting statistics 30000 Standard counting statistics 30000 Counting positioning of sample Counting positioning of standard Pulse pile-up effect - sample Pulse pile-up effect – standard Peak calculation – sample Peak calculation – standard Radiochemical separation 1.00 ± 0.06 Mass fraction of Co: 399 ng g-1 10.4 ng g-1 (k=2) (2.6%) Certified value 387 ng g-1 42 ng g-1 Rel. std. uncertainty, % 0.1 0.1 0.1 0.1 0.1 0.2 0.6 0.8 0.3 0.4 0.1 0.1 0.2 0.2 0.4 Uncertainty budget for Mo determination in NIST 1547 Peach Leaves Rel. std. uncertainty, % Mass of sample mg 150 0.1 Mass of standard mg 20 0.1 Residual blank mg 0 0.1 Neutron flux gradient 1.00± 0.025 0.1 Interfering reaction ng 2 2.1 Neutron self-shielding/scattering 0.2 Sample counting statistics Bq /counts 3000 1.8 Standard counting statistics Bq /counts 150000 0.4 Counting positioning of sample 0.3 Counting positioning of standard 0.3 Pulse pile-up effect - sample 0.1 Pulse pile-up effect – standard 0.1 Peak calculation – sample 0.2 Peak calculation – standard 0.2 Radiochemical separation 1.00 ± 0.01 0.6 Mass fraction of Mo: 58 ng g-1 3.4 ng g-1 (k=2) (5.8%) -1 -1 Certified value 60 ng g 8 ng g source of uncertainty unit value In 1998 CCQM adopted the following definition of primary method of measurement (PMM). A primary method of measurement is a method having the highest metrological qualities, whose operation can be completely described and understood, for which a complete uncertainty statement can be written down in terms of SI units. A primary direct method measures the value of an unknown without reference to a standard of the same quantity. A primary ratio method measures the value of a ratio of an unknown to a standard of the same quantity, its operation must be completely described by a measurement equation. As can be seen for the high-accuracy RNAA methods, the expanded uncertainty (k=2) varies from 2.6% for more favorable case of Co determination to 5.8% for the less favorable case of Mo determination. Such values are characteristic for the methods of the highest metrological qualities. The high-accuracy RNAA methods have the detection limits of the order of ng g-1 and provide the results with very low value of uncertainty, traceable to SI units. The methods can be fully described by measurement equations. The above statement means that the high-accuracy RNAA method fulfils the CCQM definition of a primary ratio method (PMM) and can be complementary to isotope-dilution mass spectrometry (IDMS). IDMS is the only one trace analysis method indicated by the CCQM so far as PMM. From the point of view of QA/QC of chemical measurements it is very important that at least two trace analysis PMMs based on different chemical principles can be available. Despite of the advantages of IDMS, this method has also some limitations like the all other analytical techniques. The high-accuracy RNAA method can serve for checking accuracy of the other methods used in analytical chemistry and can be applied in the certification of CRMs. Preparation and certification of CRMs • In the INCT the certification of the candidate reference materials is performed on the basis of worldwide ILC. • The applied methods of data evaluation and classification criteria have been the same with only minor modifications. • The adopted approach of data evaluation is based on outlier’s rejection procedure which uses concurrently four statistical criteria (Dixon, Grubbs, skewness and kurtosis) at the significance level of 0.05, followed by calculation of overall means of laboratory means remaining after outlier rejection, standard deviations, confidence intervals etc. Whenever possible, the process of assigning of reference values is confirm by comparison of assigned values with results obtained by the highaccuracy RNAA method. Assigning of certified value for Cd in CTA-OTL-1 High-accuracy RNAA result Certified value Range after outlier rejection Original data range 0 0,5 1 1,5 2 2,5 3 3,5 4 4,5 The same material CTA-OTL-1 was analyzed in the course of ILCs organized by the INCT • in the year 1991 (as a candidate reference material CTA-OTL-1) • and the later years 2000 and 2003 (as an unknown to participants reference material accompanying new candidate RMs). In the following figures the comparison of certified values with the values obtained by the highaccuracy RNAA method can be seen. Cd 2006 2004 2002 2000 1998 1996 1994 1992 1990 high-accuracy RNAA method 0,5 0,7 0,9 1,1 mg/kg 1,3 1,5 Co 2006 2004 2002 2000 1998 1996 1994 1992 1990 high-acuracy RNAA method 0,4 0,6 0,8 1 mg/kg 1,2 1,4 Ni 2006 2004 2002 2000 1998 1996 1994 1992 1990 high-accuracy RNAA method 3 4 5 6 mg/kg 7 8 9 Zn 2005 2000 1995 1990 40 45 50 55 mg/kg 60 65 In all figures, the good agreement of originally certified values with those that could be assigned on the basis of results from 2000 and 2003 ILCs has been obtained. The mean values with their confidence limits always overlap. The assigned values agree well with the results obtained by the high-accuracy RNAA methods. Good agreement of the mean values obtained in the period of more than 10 years, as well as with the results obtained by the high-accuracy RNAA methods shows a correctness of the adopted procedure of assigning certified values. Concluding: INAA procedures applied at the INCT enable the determination of up to 23 elements in one sample INAA procedure has been elaborated and used for testing of homogeneity of candidate RMs Many RNAA procedures have been elaborated enabling radiochemical separation of selected elements from various irradiated materials and their determination at very low level The high-accuracy RNAA methods meeting requirements of PMM have been elaborated for Cd, Co, Cu, Ni, Mo and U determination in biological materials. Thank you for your attention.