1- Define the rate of reaction and distinguish
advertisement
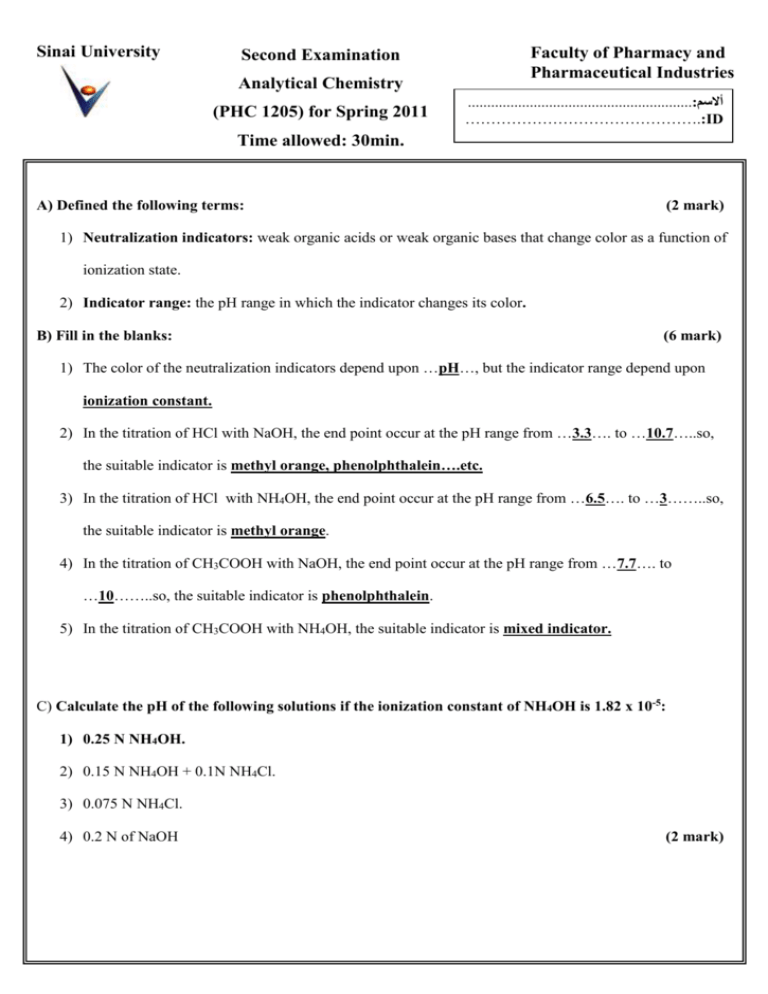
Sinai University Faculty of Pharmacy and Pharmaceutical Industries Second Examination Analytical Chemistry (PHC 1205) for Spring 2011 ..........................................................:أالسم ……………………………………….:ID Time allowed: 30min. : A) Defined the following terms: (2 mark) 1) Neutralization indicators: weak organic acids or weak organic bases that change color as a function of ionization state. 2) Indicator range: the pH range in which the indicator changes its color. B) Fill in the blanks: (6 mark) 1) The color of the neutralization indicators depend upon …pH…, but the indicator range depend upon ionization constant. 2) In the titration of HCl with NaOH, the end point occur at the pH range from …3.3…. to …10.7…..so, the suitable indicator is methyl orange, phenolphthalein….etc. 3) In the titration of HCl with NH4OH, the end point occur at the pH range from …6.5…. to …3……..so, the suitable indicator is methyl orange. 4) In the titration of CH3COOH with NaOH, the end point occur at the pH range from …7.7…. to …10……..so, the suitable indicator is phenolphthalein. 5) In the titration of CH3COOH with NH4OH, the suitable indicator is mixed indicator. C) Calculate the pH of the following solutions if the ionization constant of NH4OH is 1.82 x 10-5: 1) 0.25 N NH4OH. 2) 0.15 N NH4OH + 0.1N NH4Cl. 3) 0.075 N NH4Cl. 4) 0.2 N of NaOH (2 mark) 1) 0.25 N NH4OH. 1 1 pH 14 pK b pC b 2 2 1 1 pH 14 x 4.74 x 0.60 11.32 2 2 2) 0.15 N NH4OH + 0.1N NH4Cl. pH 14 - pK b log [base] [salt] 0.15 9.44 0.1 pH 14 - 4.74 log pH 1 1 1 pK w pK b pC s 2 2 2 pH 1 1 1 x 14 x 4.74 x 1.12 5.19 2 2 2 3) 0.075 N NH4Cl. 4) 0.2 N of NaOH pOH = - log 0.2 = 0.7 pH = 14 – 0.7 = 13.30








