secondary phloem - The Virtual Plant
advertisement

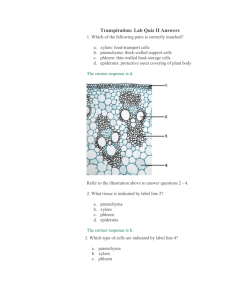
Oldenburgia grandis, Botany RU a quick lesson in embryogenesis sporophyte seed 2N MEIOSIS FERTILIZATION N megaspores sperm egg megagametophyte microgametophyte The triangle of differentiation microspores Where do we start? gametogenesis seed embryo totipotency [differentiation, maturation] initiation of SAM, RAM (polarity?) [differentiation] cell division, formation of axes expansion growth axis & coleoptiles form ----------------- Seed to plant Seed monocotyledon dicotyledon activation of SAM & RAM Embryo proper of Capsella cell division (genes, hormones) procambium expansion, cell elongation graviperception phototropism Post-Germination: Rapid growth 2h 2h 20min Growth in this bean plant is due in part to cell division, but, to a larger extent, DUE to longitudinal elongation of cells – all controlled by genes. 2h 40 min Apical organization Organization in plants is dependent upon programmed, controlled cell division, followed by growth, further cell division and ultimately, differentiation. Programmed and controlled cell division occurs within the domain of the vegetative apex. the apex All the tissues within the apex differentiate rapidly. By about 150 µm, cells within the apical region are starting to differentiate. In the pine apex (above), you can see developing leaflets. The Coleus apex to the right, shows rapidly developing leaflets beneath the apical dome. cell division Cell division is responsible for the formation of all cells and tissues in the primary plant body as well as in the secondary plant body. Cell source apical and sub apical primary division undifferentiated generative source protoderm primary lineage epidermis Secondary cell lineage apical meristem provascular tissue primary xylem primary phloem fascicular cambium ground meristem pith cortex the ground tissue cortex STOP parenchyma filling tissue collenchyma sclerenchyma mechanical, supportive the secondary lineage COMPLETE RING OF CAMBIUM fascicular cambium ASSOCIATED WITH THE VASCULAR BUNDLE ONLY vascular cambium secondary xylem secondary phloem cork cambium the secondary protective lineage subepidermal layers the cork cambium (bark layer) phellogen phellem the periderm a protective barrier phelloderm Development of the periderm subepidermal layers The first periderm is formed just beneath the epidermis phellogen phellem a waterproof, fireproof insulator phelloder m phellem phellogen phelloderm primary organization ground meristem PITH CORTEX fascicular cambium interfascicular cambium vascular cambium secondary xylem secondary phloem cork cambium Click for Filling spaces notes phellem/cork cambium primary mechanical tissues ground meristem ground meristem PITH CORTEX CORTEX collenchyma interfascicular cambium PITH phellem/cor k cambium sclerenchyma collenchyma (rare) sclerenchyma development of the vascular cambium fascicular cambium fascicular cambium fusiform initials vascular cambium secondary xylem secondary phloem axial xylem ray initials axial phloem cork cambium xylem rays to cambial derivatives notes pages phloem rays initial axial cambial division Cell division within the ray and fusiform initials results in the formation of derivative cells that are placed either on the outside of the mother cell, in which case they add to the secondary phloem, or on the inside endarch to) the mother cell, thus adding to the secondary xylem Cell source The apical meristem is the principle source of new cells in the primary as well as within the secondary plant body. All cell division linked to vegetative growth, involves mitosis, and, as a result, the cells that are produced are exact copies of each other. Lineage depends on the position of the initial within the meristem. the periderm a protective barrier During secondary growth, the diameter of stems and roots increases rapidly, which results in tension and splitting of the existing dermal tissues, which subsequently, will stretch and become disrupted. The generative layer of the first periderm (phellogen) is initiated within parenchymatous elements in the outer cortex of stems and roots. It offers protection from invasion by insects, pathogens and fungi. As the stem or root continues to increase in diameter, so successive periderms are formed. These are formed within the secondary phloem. The periderm is a natural waterproof, fireproof insulator. Filling spaces Within all plants the primary packaging tissues are composed of cells that either fill in spaces, or support other areas of the stem, root or leaf. Thus, the parenchymatic elements that are produced (and have lineage back to the apical meristems) are produced from what is termed the ground meristem. In simple terms, the ground meristem is that region of a shoot or root apical meristem that is NOT involved in the production of vascular tissue. cambial derivatives The vascular cambium is the source of all needed (secondary) differentiation in plants. It contains two systems, the secondary xylem, and the secondary phloem tissue. Each of these tissues is complex, and is developed and has evolved for specific functions – the xylem for the transport of water and water soluble molecules, the phloem for the transport of assimilated, and the, which consist of sugars and related carbohydrates translocated in water. Physiologically, the transport xylem is dead at maturity, has secondarily-lignified cell walls, and functions under extreme negative pressure potentials. Transport phloem on the other hand, contains a majority of living cells, with specialized sieve elements, which are geared for rapid, long-distance translocation of the assimilated carbohydrate pool. These transport elements, have thickened walls, are living at maturity and function under a high positive pressure potential. click here for the next page transport functionality The xylem and phloem conduits form axial tubes. These tubes facilitate rapid, long-distance movement of water and dissolved materials. It follows therefore that the fascicular cambial derivatives that form these transport cells are longer than they are wide, and that the cells will, depending on position form either xylem or phloem. click here for cambial derivatives click the need for lateral communication back the need for lateral communication As the secondary plant body enlarges, so the carbohydrate conducting, and water transporting systems become laterally spatially and physiologically further removed from each other. The core of a stem or root, for example, may well contain a number of living cells, that not only require water and a supply of assimilate and other carbohydrates, in order to maintain their functional state. If this does not happen or if the supply is cut off for some reason, then the core will die. Lateral communication, and the production of these cells in the lateral communication pathway, is due to the activity of specialised cambial cells, called the ray cells. These cells are sort, often cubic in shape and the produce rows (files) of parenchymatous living cells, that interconnect the phloem with the inner xylem core, thereby facilitating exchange of carbohydrate inwards, and water outwards in the living plant. zipping organ formation Arabidopsis class III homeodomain-leucine zipper (HD-Zip III) proteins play overlapping, distinct, and antagonistic roles in key aspects of development that have evolved during land plant evolution. Plant signals Figure 1 Model of how CLASS III HD-ZIP1 and KANADI activities pattern lateral organs and vasculature. A centrally derived signal (red) activates CLASS III HD-ZIP genes, whose activity is antagonistic with that of KANADI activity. Both KANADI and MIR165/166 negatively regulate CLASS III HD-ZIP genes, (relationship between the two is not presently known). In lateral organs, CLASS III HD-ZIP activity promotes adaxial fates and KANADI activity promotes abaxial fates. In the vascular bundles, interactions between the two gene classes pattern the arrangement of xylem and phloem tissues. The vascular bundle shown is already differentiated, but the initial patterning events likely occur just below the apical meristem where provascular cells are being specified. –––––––––––––––-– 1Class III homeodomain-leucine zipper proteins See http://www.nature.com/nrm/journal/v5/n5/full/nrm1364.html Scarecrow The formation of the cortex and endodermal layers in the Arabidopsis root requires two asymmetric divisions. In the first, an anticlinal division of the cortex/endodermal initial generates two cells with different developmental potentials. One will continue to function as an initial, the other undergoes a periclinal division to generate the first cells in the endodermal and cortex cell files. This second asymmetric division is eliminated in the scr mutant, resulting in a single cell layer instead of two. Laura Di Laurenzio et al., Cell, Vol. 86, 423–433, August 9, 1996, Copyright ã1996 by Cell Press Homework: Spend a bit of time researching other gene systems (in Arabidopsis, or higher plants) that are involved in SAM or RAM development, expression of morphology, size and shape. Insert into a word doc, CITE the references as well please and send them to me – I will collate and redistribute useful information back to you via RUConnected end