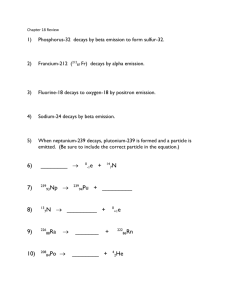
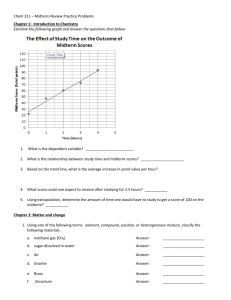
Isotopes of Cerium Notable Isotopes 134Ce [76 neutrons
advertisement

Isotopes of Cerium Notable Isotopes 134 Ce [76 neutrons] Abundance: synthetic Half life: 3.16 days [ Electron Capture ] Decay Energy: 0.500MeV Decays to 136 134 La. Ce [78 neutrons] Abundance: 0.19% Stable with 78 neutrons 138 Ce [80 neutrons] Abundance: 0.25% Stable with 80 neutrons 139 Ce [81 neutrons] Abundance: synthetic Half life: 137.640 days [ Electron Capture ] Decay Energy: 0.278MeV Decays to 140 139 La. Ce [82 neutrons] Abundance: 88.48% Stable with 82 neutrons 141 Ce [83 neutrons] Abundance: synthetic Half life: 32.501 days [ beta- ] Decay Energy: 0.581MeV Decays to 142 141 Pr. Ce [84 neutrons] Abundance: 11.08% Half life: 5 x 1016 years [ beta- ] Decay Energy: ?MeV Decays to 144 142 Nd. Ce [86 neutrons] Abundance: synthetic Half life: 284.893 days [ beta- ] Decay Energy: 0.319MeV Decays to 144 Pr. Cerium Compounds Ammonium cerium(IV) nitrate Cerium(IV) oxide Ammonium cerium(IV) nitrate (NH4)2Ce(NO3)6 An important component of Chrome etchant, a material that is used in the production of liquid crystal displays (LCDs). Cerium(IV) oxide CeO2 It is used in ceramics, to polish glass, and to sensitize photosensitive glass. It is also used in the walls of self-cleaning ovens. Reactions of Cerium Reactions with water Cerium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form cerium hydroxide and hydrogen gas. 2Ce(s) + 6H2O(g) 2Ce(OH)3(aq) + 3H2(g) Reactions with air Cerium tarnishes slowly in air and burns readily to form cerium (IV) oxide. Ce + O2 CeO2 Reactions with halogens Cerium metal reacts with all the halogens to form cerium(III) halides. 2Ce(s) + 3F2(g) 2CeF3(s) 2Ce(s) + 3Cl2(g) 2CeCl3(s) 2Ce(s) + 3Br2(g) 2CeBr3(s) 2Ce(s) + 3I2(g) 2CeI3(s) Reactions with acids Cerium dissolves readily in dilute sulphuric acid to form solutions containing the colourless aquated Ce(III) ion together with hydrogen gas. 2Ce(s) + 3H2SO4(aq) 2Ce3+(aq) + 3SO42-(aq) + 3H2(g) Reduction Potentials Balanced half-reaction Ce3+ + 3eCe(OH)2 Ce(OH) 2+ 3+ E0 / V Ce(s) -2.48 + 2H + e + Ce - +H +e + Ce - 3+ 3+ + 2H2O + H2O Ce(ClO4)62- + e- Ce3+ + 6ClO4- Ce(NO3)6 - - 2- +e Ce Ce(SO4)32- +e Ce 3+ 3+ +1.73 +1.71 +1.70 + 6NO3- +1.61 + 3SO42- +1.44 Occurrence and Production of Cerium Occurrence Cerium is the most abundant of the rare earths elements, making up about 0.0046% of the Earth's crust by weight. It is found in a number of minerals including allanite (also known as orthite)-(Ca, Ce, La, Y)2(Al, Fe)3(SiO4)3(OH), monazite (Ce, La, Th, Nd, Y)PO4, bastnasite(Ce, La, Y)CO3F, hydroxylbastnasite (Ce, La, Nd)CO3(OH, F), rhabdophane (Ce, La, Nd)PO4 - H2O, zircon (ZrSiO4), and synchysite Ca(Ce, La, Nd, Y)(CO3)2F. Monazite and bastnasite are presently the two most important sources of cerium. Cerium is most often prepared via an ion exchange process that uses monazite sands as its cerium source. Large deposits of monazite, allanite, and bastnasite will supply cerium, thorium, and other rare-earth metals for many years to come. Praseodymium [Pr] CAS-ID: 7440-10-0 An: 59 N: 82 Am: 140.90765(2) g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white, yellowish tinge Classification: Metallic Boiling Point: 3563K (3290°C) Melting Point: 1208K (935°C) Density: 6.77g/cm3 Discovery Information Who: C.F. Aver von Welsbach When: 1885 Where: Austria Name Origin Greek: prasios (green) and didymos (twin); from its green salts. "Praseodymium" in different languages. Sources Can be found in rare earth minerals such as bastnasite and monazite. Obtained from the same salts as neodymium. Primary producers are the USA, Brazil, India, Sri Lank and Australia. Annual production is around 2400 tons. Abundance Universe: 0.002 ppm (by weight) Sun: 0.001 ppm (by weight) Carbonaceous meteorite: 0.1 ppm Earth's Crust: 8.7 ppm Seawater: Atlantic surface: 4 x 10-7 ppm Atlantic deep: 7 x 10-7 ppm Pacific surface: 4.4 x 10-7 ppm Pacific deep: 1 x 10-6 ppm Uses Praseodymium forms the core of carbon arc lights which are used in the motion picture industry for studio lighting and projector lights. Used for colouring glass and ceramic glazes. Also used with neodymium to make lenses for glass maker's goggles because they filter out the yellow light present in glass blowing. It is alloyed with magnesium to create high strength metals. History In 1841, Mosander extracted the rare earth didymium from lanthana. In 1874, Per Teodor Cleve concluded that didymium was in fact two elements, and in 1879, Lecoq de Boisbaudran isolated a new earth, samarium, from didymium obtained from the mineral samarskite. In 1885, the Austrian chemist baron Carl Auer von Welsbach separated didymium into two elements, praseodymium and neodymium, which gave salts of different colours. Leo Moser investigated the use of praseodymium in glass colouration in the late 1920s. The result was a yellow-green glass given the name "Prasemit". However, a similar colour could be achieved with colourants costing only a minute fraction of what praseodymium cost in the late 1920s, such that the colour was not popular, few pieces were made, and examples are now extremely rare. Moser also blended praseodymium with neodymium to produce "Heliolite" glass ("Heliolit" in German), which was more widely accepted. The first enduring commercial use of praseodymium, which continues today, is in the form of a yellow-orange stain for ceramics, "Praseodymium Yellow", which is a solid-solution of praseodymium in the zirconium silicate (zircon) lattice. This stain has no hint of green in it. By contrast, at sufficiently high loadings, praseodymium glass is distinctly green, rather than pure yellow. Praseodymium has historically been a rare earth whose supply has exceeded demand. Unwanted as such, much praseodymium has been marketed as a mixture with lanthanum and cerium, or "LCP" for the first letters of each of the constituents, for use in replacing the traditional lanthanoid mixtures that were inexpensively made from monazite or bastnaesite. LCP is what remains of such mixtures, after the desirable neodymium, and all the heavier, rarer and more valuable lanthanoids have been removed, by solvent extraction. However, as technology progresses, praseodymium has been found possible to incorporate into neodymium-iron-boron magnets, thereby extending the supply of the much in demand neodymium. So LC is starting to replace LCP as a result. Notes Dr. Matthew Sellars of the Laser Physics Centre at the Australian National University in Canberra, Australia slowed down a light pulse to a few hundred meters per second using Praseodymium mixed with silicate crystal. Hazards Like all rare earth elements, praseodymium is of low to moderate toxicity. Praseodymium has no known biological role. Isotopes of Praseodymium Notable Isotopes 141 Pr [82 neutrons] Abundance: 100% Stable with 82 neutrons 142 Pr [83 neutrons] Abundance: synthetic Half life: 19.12 hours [ beta- ] [ Electron Capture ] Decay Energy: 2.1620.745MeV Decays to 143 142 Nd142Ce. Pr [84 neutrons] Abundance: synthetic Half life: 13.57 days [ beta- ] Decay Energy: 0.934MeV Decays to 143 Nd. Reactions of Praseodymium Reactions with water Praseodymium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form praseodymium hydroxide and hydrogen gas. 2Pr(s) + 6H2O(g) 2Pr(OH)3(aq) + 3H2(g) Reactions with air Praseodymium tarnishes slowly in air and burns readily to form a praseodymium oxide which has the approximate formula Pr6O11. 12Pr(s) + 11O2(g) 2Pr6O11(s) Reactions with halogens Praseodymium reacts with all the halogens to form praseodymium(III) halides. 2Pr(s) + 3F2(g) 2PrF3(s) 2Pr(s) + 3Cl2(g) 2PrCl3(s) 2Pr(s) + 3Br2(g) 2PrBr3(s) 2Pr(s) + 3I2(g) 2PrI3(s) Reactions with acids Praseodymium dissolves readily in dilute sulphuric acid to form solutions containing the green aquated Pr(III) ion together with hydrogen gas. 2Pr(s) + 3H2SO4(aq) 2Pr3+(aq) + 3SO42-(aq) + 3H2(g) 141 Pr Neodymium [Nd] CAS-ID: 7440-00-8 An: 60 N: 84 Am: 144.24 g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white, yellowish tinge Classification: Metallic Boiling Point: 3373K (3100°C) Melting Point: 1297K (1024°C) Density: 7.01g/cm3 Discovery Information Who: C.F. Aver von Welsbach When: 1925 Where: Austria Name Origin Greek: neos (new) didymos (twin). "Neodymium" in different languages. Sources Neodymium is never found in nature as the free element. It occurs in ores such as monazite sand and bastnasite. Primary producers are the USA, Brazil, India, Sri Lanka and Australia. Around 7300 tons are produced annually. Abundance Universe: 0.01 ppm (by weight) Sun: 0.003 ppm (by weight) Carbonaceous meteorite: 0.51 ppm Earth's Crust: 33 ppm Seawater: Atlantic surface: 1.8 x 10-6 ppm Atlantic deep: 3.2 x 10-6 ppm Pacific surface: 1.8 x 10-6 ppm Pacific deep: 4.8 x 10-6 ppm Uses Used in ceramics to colour glazes, and for special lens with praseodymium. Also to produce bright purple glass and special glass that filters infrared radiation. Neodymium is used in very powerful permanent magnets - Nd2Fe14B. These magnets are cheaper and also stronger than samarium-cobalt magnets. Neodymium magnets appear in products such as in-ear headphones and computer hard drives. History Neodymium was discovered by Baron Carl Auer von Welsbach, an Austrian chemist, in Vienna in 1885. He separated neodymium, as well as the element praseodymium, from a material known as didymium by means of fractional crystallization of the double ammonium nitrate tetrahydrates from nitric acid, while following the separation by spectroscopic analysis; however, it was not isolated in relatively pure form until 1925. The name neodymium is derived from the Greek words neos, new, and didymos, twin. Neodymium is frequently misspelled as neodynium. Double nitrate crystallization was the means of commercial neodymium purification until the 1950's. The Lindsay Chemical Division of American Potash and Chemical Corporation, at one time the largest producer of rare earths in the world, offered neodymium oxide purified in this manner in grades of 65%, 85% and 95% purity, at prices ranging from approximately 2 to 20 dollars per pound (in 1960 dollars). Lindsay was the first to commercialize large-scale ion-exchange purification of neodymium, using the technology developed by Frank Spedding at Iowa State University/Ames Laboratory; one pound of their 99% oxide was priced at $35 in 1960; their 99.9% grade only cost 5 dollars more. Starting in the 1950's, high purity (e.g. 99+%) neodymium was primarily obtained through an ion exchange process from monazite sand ((Ce,La,Th,Nd,Y)PO4), a material rich in rare earth elements. The metal itself is obtained through electrolysis of its halide salts. Currently, most neodymium is extracted from bastnaesite, (Ce,La,Nd,Pr)CO3F, and purified by solvent extraction. Ionexchange purification is reserved for preparing the highest purities (typically greater than 4N). (When Molycorp first introduced their 98% grade of neodymium oxide in 1965, made by solvent extraction from Mountain Pass California bastnaesite, it was priced at 5 dollars per pound, for small quantities. Lindsay soon discontinued operations.) The evolving technology, and improved purity of commercially available neodymium oxide was reflected in the appearance of neodymium glass made therefrom, that resides in collections today. Early Moser pieces, and other neodymium glass made in the 1930's, have a more reddish or orange tinge than modern versions, which are more cleanly purple, due to the difficulties in removing the last traces of praseodymium, when the fractional crystallization technology had to be relied on. Notes Size and strength of volcanic eruption can be predicted by scanning for neodymium isotopes. Small and large volcanic eruptions produce lava with different neodymium isotope composition. From the composition of isotopes, scientists predict how big the coming eruption will be, and use this information to warn residents of the intensity of the eruption. Hazards Neodymium metal dust is a combustion and explosion hazard. Neodymium dust and salts are very irritating to the eyes and mucous membranes, and moderately irritating to skin. Breathing the dust can cause lung embolisms, and accumulated exposure damages the liver. Neodymium also acts as an anticoagulant, especially when given intravenously. Neodymium compounds, like all rare earth metals, are of low to moderate toxicity; however its toxicity has not been thoroughly investigated. Isotopes of Neodymium Notable Isotopes 142 Nd [82 neutrons] Abundance: 27.13% Stable with 82 neutrons 143 Nd [83 neutrons] Abundance: 12.18% Stable with 83 neutrons 144 Nd [84 neutrons] Abundance: 23.8% Half life: 2.29 x 1015 years [ Alpha Decay ] Decay Energy: 1.905MeV Decays to 145 140 Ce. Nd [85 neutrons] Abundance: 8.3% Stable with 85 neutrons 146 Nd [86 neutrons] Abundance: 17.19% Stable with 86 neutrons 148 Nd [88 neutrons] Abundance: 5.76% Stable with 88 neutrons 150 Nd [90 neutrons] Abundance: 5.64% Half life: 1.1 x 1019 years [ Double beta decay ] Decay Energy: 3.367MeV Decays to Sm. 150 Reactions of Neodymium Reactions with water Neodymium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form neodymium hydroxide and hydrogen gas. 2Nd(s) + 6H2O(g) 2Nd(OH)3(aq) + 3H2(g) Reactions with air Neodymium tarnishes slowly in air and burns readily to form neodymium (III) oxide. 4Nd + 3O2 2Nd2O3 Reactions with halogens Neodymium reacts with all the halogens to form neodymium(III) halides. 2Nd(s) + 3F2(g) 2NdF3(s) 2Nd(s) + 3Cl2(g) 2NdCl3(s) 2Nd(s) + 3Br2(g) 2NdBr3(s) 2Nd(s) + 3I2(g) 2NdI3(s) Reactions with acids Neodymium dissolves readily in dilute sulphuric acid to form solutions containing the lilac aquated Nd(III) ion together with hydrogen gas. 2Nd(s) + 3H2SO4(aq) 2Nd3+(aq) + 3SO42-(aq) + 3H2(g) Promethium [Pm] CAS-ID: 7440-12-2 An: 61 N: 84 Am: [145] g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: metallic Classification: Metallic Boiling Point: 3273K (3000°C) Melting Point: 1373K (1100°C) Density: 7.26g/cm3 Discovery Information Who: J.A. Marinsky, L.E. Glendenin, C.D. Coryell When: 1945 Where: United States Name Origin From Prometheus who stole the fire of the sky and gave it to mankind. "Promethium" in different languages. Sources Does not occur naturally. Found among fission products of uranium, thorium, and plutonium. Uses Used as a radiation source in thickness gauges, photoelectric cells and hold potential as a heat source for auxiliary power in satellites. In a nuclear battery in which photocells convert the light into electric current, yielding a useful life of about five years using 147Pm. History The existence of promethium was first predicted by Bohuslav Brauner in 1902; this prediction was supported by Henry Moseley in 1914, who found a gap for a missing element which would have atomic number 61, but was unknown (however, Moseley of course had no sample of the element to verify this). Several groups claimed to have produced the element, but they could not confirm their discoveries because of the difficulty of separating promethium from other elements. Promethium was first produced and proved to exist at Oak Ridge National Laboratory (ORNL) in 1945 by Jacob A. Marinsky, Lawrence E. Glendenin and Charles D. Coryell by separation and analysis of the fission products of uranium fuel irradiated in the Graphite Reactor; however, being too busy with defense-related research during World War II, they did not announce their discovery until 1947. The name promethium is derived from Prometheus in Greek mythology, who stole the fire of the sky and gave it to mankind. The name was suggested by Grace Mary Coryell, Charles Coryell's wife, who felt that they were stealing fire from the gods. In 1963, ion-exchange methods were used at ORNL to prepare about 10 grams of promethium from nuclear reactor fuel processing wastes. Today, promethium is still recovered from the byproducts of uranium fission; it can also be produced by bombarding 146Nd with neutrons, turning it into 147Nd which decays into 147Pm through beta decay with a half-life of 11 days. Notes Promethium is also the name of a fictional element in the DC Universe; writer Marv Wolfman claims to have been unaware of the existence of a real substance by that name at the time he wrote the original script featuring the name. Hazards Promethium must be handled with great care because of its high radioactivity. Isotopes of Promethium Notes Promethium has no stable isotopes. Notable Isotopes 145 Pm [84 neutrons] Abundance: synthetic Half life: 17.7 years [ Electron Capture ] Decay Energy: 0.163MeV Decays to 146 145 Nd. Pm [85 neutrons] Abundance: synthetic Half life: 5.53 years [ Electron Capture ] Decay Energy: 1.472MeV Decays to 146 Nd. Half life: 5.53 years [ beta- ] Decay Energy: 1.542MeV Decays to 147 Sm. 146 Pm [86 neutrons] Abundance: synthetic Half life: 2.6234 years [ beta- ] Decay Energy: 0.224MeV Decays to Sm. 147 Reactions of Promethium Reactions with acids Promethium dissolves readily in dilute sulphuric acid to form solutions containing the pink aquated Pm(III) ion together with hydrogen gas. 2Pm(s) + 3H2SO4(aq) 2Pm3+(aq) + 3SO42-(aq) + 3H2(g) Samarium [Sm] CAS-ID: 7440-19-9 An: 62 N: 88 Am: 150.36 g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 2076K (1803°C) Melting Point: 1345K (1072°C) Density: 7.52g/cm3 Discovery Information Who: Paul emile Lecoq de Boisbaudran When: 1879 Where: France Name Origin From the mineral samarskite, named after a Russian mine official, Colonel Samarski. "Samarium" in different languages. Sources Never found free in nature. Samarium is found in many minerals, including bastnasite, monazite and samarskite. Primary producers are the USA, Brazil, India, Sri Lanka and Australia. Around 700 tons are produced annually. Abundance Universe: 0.005 ppm (by weight) Sun: 0.001 ppm (by weight) Carbonaceous meteorite: 0.17 ppm Earth's Crust: 6 ppm Seawater: Atlantic surface: 4 x 10-7 ppm Atlantic deep: 6.4 x 10-7 ppm Pacific surface: 4 x 10-7 ppm Pacific deep: 1 x 10-6 ppm Uses Used in carbon-arc lighting, permanent magnets, lasers, alloys, headphones and as an absorber in nuclear reactors. Samarium oxide is used in optical glass to absorb infrared. Samarium-Cobalt magnets; SmCo5 is used in making a new permanent magnet material with the highest resistance to demagnetization of any known material. History Samarium was first discovered spectroscopically in 1853 by Swiss chemist Jean Charles Galissard de Marignac by its sharp absorption lines in didymium, and isolated in Paris in 1879 by French chemist Paul Emile Lecoq de Boisbaudran from the mineral samarskite ((Y,Ce,U,Fe)3(Nb,Ta,Ti)5O16). Although samarskite was first found in the Urals, by the late 1870s a new deposit had been located in North Carolina, and it was from that source that the samarium-bearing didymium had originated. The samarskite mineral was named after Vasili Samarsky-Bykhovets, the Chief of Staff (Colonel) of the Russian Corps of Mining Engineers in 1845-1861. The name of the element is derived from the name of the mineral, and thus traces back to the name Samarsky-Bykhovets. In this sense samarium was the first chemical element to be named after a living person. Prior to the advent of ion-exchange separation technology in the 1950s, samarium had no commercial uses in pure form. However, a by-product of the fractional crystallization purification of neodymium was a mixture of samarium and gadolinium that acquired the name of "Lindsay Mix" after the company that made it. This material is thought to have been used for nuclear control rods in some of the early nuclear reactors. Nowadays, a similar commodity product goes under the name of "SamariumEuropium-Gadolinium" concentrate (or SEG concentrate). This is prepared by solvent extraction from the mixed lanthanoids extracted from bastnaesite (or monazite). Since the heavier lanthanoids have the greater affinity for the solvent used, they are easily extracted from the bulk using relatively small proportions of solvent. Not all rare earth producers who process bastnaesite do so on large enough scale to continue onward with the separation of the components of SEG, which typically makes up only one or two percent of the original ore. Such producers will therefore be making SEG with a view to marketing it to the specialized processors. In this manner, the valuable europium content of the ore is rescued for use in phosphor manufacture. Samarium purification follows the removal of the europium. Currently, being in oversupply, samarium oxide is less expensive on a commercial scale than its relative abundance in the ore might suggest. Isotopes of Samarium Notable Isotopes Sm [82 neutrons] 144 Abundance: 3.07% Stable with 82 neutrons Sm [84 neutrons] 146 Abundance: synthetic Half life: 1.03 x 108 years [ Alpha Decay ] Decay Energy: 2.529MeV Decays to 142 Nd. Sm [85 neutrons] 147 Abundance: 14.99% Half life: 1.06 x 1011 years [ Alpha Decay ] Decay Energy: 2.310MeV Decays to 143 Nd. Sm [86 neutrons] 148 Abundance: 11.24% Half life: 7 x 1015 years [ Alpha Decay ] Decay Energy: 1.986MeV Decays to 144 Nd. Sm [87 neutrons] 149 Abundance: 13.82% Half life: 2 x 1015 years [ Alpha Decay ] Decay Energy: 1.870MeV Decays to 145 Nd. Sm [88 neutrons] 150 Abundance: 7.38% Stable with 88 neutrons Sm [90 neutrons] 152 Abundance: 26.75% Stable with 90 neutrons Sm [92 neutrons] 154 Abundance: 22.75% Stable with 92 neutrons Reactions of Samarium Reactions with water Samarium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form samarium hydroxide and hydrogen gas. 2Sm(s) + 6H2O 2Sm(OH)3(aq) + 3H2(g) Reactions with air Samarium tarnishes slowly in air and burns readily to form samarium (III) oxide. 4Sm(s) + 3O2(g) 2Sm2O3(s) Reactions with halogens Samarium reacts with all the halogens to form samarium(III) halides. 2Sm(s) + 3F2(g) 2SmF3(s) 2Sm(s) + 3Cl2(g) 2SmCl3(s) 2Sm(s) + 3Br2(g) 2SmBr3(s) 2Sm(s) + 3I2(g) 2SmI3(s) Reactions with acids Samarium dissolves readily in dilute sulphuric acid to form solutions containing the yellow aquated Sm(III) ion together with hydrogen gas. 2Sm(s) + 3H2SO4(aq) 2Sm3+(aq) + 3SO42-(aq) + 3H2(g) Europium [Eu] CAS-ID: 7440-53-1 An: 63 N: 89 Am: 151.964 (1) g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 1800K (1527°C) Melting Point: 1099K (826°C) Density: 5.244g/cm3 Discovery Information Who: Eugene Demarcay When: 1901 Where: France Name Origin From Europe. "Europium" in different languages. Sources It is never found in nature as a free element. There are many minerals that contain europium, the important of these are bastnasite and monazite. Primary mining locations are the USA, Brazil, India, Sri Lanka, Australia and China. Annual production is around 400 tons. Abundance Universe: 0.0005 ppm (by weight) Sun: 0.0005 ppm (by weight) Carbonaceous meteorite: 0.006 ppm Earth's Crust: 2.1 ppm Seawater: Atlantic surface: 9 x 10-8 ppm Atlantic deep: 1.5 x 10-7 ppm Pacific surface: 1 x 10-7 ppm Pacific deep: 2.7 x 10-7 ppm Uses Europium oxide (Eu2O3) along with yttrium oxide are used to make red phosphors for colour televisions. A salt of Europium is a component of the newer phosphorescent powders and paints, some of which will glow for days after a few minutes of exposure to light. A salt of Europium is a component of the newer phosphorescent powders and paints, some of which will glow for days after a few minutes of exposure to light. Europium fluorescence is used to interogate biomolecular interactions in drug-discovery screens. It is also used in the anti-counterfeiting phosphors in Euro banknotes. History Europium was first found by Paul Emile Lecoq de Boisbaudran in 1890, who obtained basic fraction from samarium-gadolinium concentrates which had spectral lines not accounted for by samarium or gadolinium; however, the discovery of europium is generally credited to French chemist Eugene-Anatole Demarcay, who suspected samples of the recently discovered element samarium were contaminated with an unknown element in 1896 and who was able to isolate europium in 1901. When the europium-doped yttrium orthovanadate red phosphor was discovered in the early 1960s, and understood to be about to cause a revolution in the colour television industry, there was a mad scramble for the limited supply of europium on hand among the monazite processors. (Typical europium content in monazite was about 0.05%.) Luckily, Molycorp, with its bastnaesite deposit at Mountain Pass California, whose lanthanoids had an unusually "rich" europium content of 0.1%, was about to come online and provide sufficient europium to sustain the industry. Prior to europium, the colour-TV red phosphor was very weak, and the other phosphor colours had to be muted, to maintain colour balance. With the brilliant red europium phosphor, it was no longer necessary to mute the other colours, and a much brighter colour TV picture was the result. Europium has continued in use in the TV industry ever since, and, of course, also in computer monitors. California bastnaesite now faces stiff competition from Bayan Obo, China, with an even "richer" europium content of 0.2%. Frank Spedding, celebrated for his development of the ion-exchannge technology that revolutionized the rare earth industry in the mid-1950's once related the story of how, in the 1930's, he was lecturing on the rare earths when an elderly gentleman approached him with an offer of a gift of several pounds of europium oxide. This was an unheard-of quantity at the time, and Spedding did not take the man seriously. However, a package duly arrived in the mail, containing several pounds of genuine europium oxide. The elderly gentleman had turned out to be the Dr. McCoy who had developed a famous method of europium purification involving redox chemistry. Notes Europium is the most reactive of the rare earth elements; it quickly oxidizes in air, and resembles calcium in its reaction with water. It is about as hard as lead and quite ductile. Hazards As dust, europium presents a fire and explosion hazard. Isotopes of Europium Notable Isotopes 150 Eu [87 neutrons] Abundance: synthetic Half life: 36.9 years [ Electron Capture ] Decay Energy: 2.261MeV Decays to Sm. 150 151 Eu [88 neutrons] Abundance: 47.8% Stable with 88 neutrons 152 Eu [89 neutrons] Abundance: synthetic Half life: 13.516 years [ Electron Capture ] Decay Energy: 1.874MeV Decays to Sm. 152 Half life: 13.516 years [ beta- ] Decay Energy: 1.819MeV Decays to 153 152 Gd. Eu [90 neutrons] Abundance: 52.2% Stable with 90 neutrons Reactions of Europium Reactions with water Europium is quite electropositive and reacts slowly with cold water and fairly quickly with hot water to form europium hydroxide, and hydrogen gas. 2Eu(s) + 6H2O(g) 2Eu(OH)3(aq) + 3H2(g) Reactions with air Europium metal burns readily in air to form europium(III) oxide. 4Eu + 3O2 2Eu2O3 Reactions with halogens Europium metal reacts with all the halogens to form europium(III) halides. 2Eu(s) + 3F2(g) 2EuF3(s) 2Eu(s) + 3Cl2(g) 2EuCl3(s) 2Eu(s) + 3Br2(g) 2EuBr3(s) 2Eu(s) + 3I2(g) 2EuI3(s) Reactions with acids Europium metal dissolves readily in dilute sulphuric acid to form solutions containing the very pale pink Eu(III) ion together with hydrogen gas. 2Eu(s) + 3H2SO4(aq) 2Eu3+(aq) + 3SO42-(aq) + 3H2(g) Gadolinium [Gd] CAS-ID: 7440-54-2 An: 64 N: 93 Am: 157.25 g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 3523K (3250°C) Melting Point: 1585K (1312°C) Superconducting temperature: 1.083K (-272.067°C) Density: 7.90g/cm3 Discovery Information Who: Jean de Marignac When: 1880 Where: Switzerland Name Origin Named after the Finnish chemist and geologist Johan Gadolin. "Gadolinium" in different languages. Sources Gadolinium is never found in nature in elemental form. It is obtained from many rare minerals such as bastnasite, monazite and trace amounts in gadolinite. Primary mining deposits are located in the USA, Brazil, India, Sri Lanka, Australia and Chine. Annual production is around 400 tons. Abundance Universe: 0.002 ppm (by weight) Sun: 0.002 ppm (by weight) Carbonaceous meteorite: 0.23 ppm Earth's Crust: 7.7 ppm Seawater: Atlantic surface: 5.2 x 10-7 ppm Atlantic deep: 9.3 x 10-7 ppm Pacific surface: 6 x 10-7 ppm Pacific deep: 1.5 x 10-6 ppm Uses Compounds of gadolinium are used in making phosphors for colour TV tubes and in the manufacture of compact discs and computer memory. Gallium Gadolinium Garnet (Gd3Ga5O12) is a material with good optical properties, and is used in fabrication of various optical components and as substrate material for magneto-optical films. Gadolinium is used for making gadolinium yttrium garnets, which have microwave applications. Solutions of organic gadolinium complexes are used as intravenous radiocontrast agents to enhance images in medical magnetic resonance imaging. Because of their paramagnetic properties, gadolinium compounds are used in magnetic resonance imaging (MRI). History In 1880, Swiss chemist Jean Charles Galissard de Marignac observed spectroscopic lines due to gadolinium in samples of didymium and gadolinite; French chemist Paul Emile Lecoq de Boisbaudran separated gadolinia, the oxide of Gadolinium, from Mosander's yttria in 1886. The element itself was isolated only recently. In older literature the natural form of the element is often called an "earth", meaning that element came from the Earth. Accordingly - Gadolinium is the element that comes from the earth, gadolinia. Earths are compounds of the element and one or more other elements. Two common combining elements are oxygen and sulfur. For example, gadolinia contains gadolinium oxide (Gd2O3). Notes Gadolinium becomes superconductive below a critical temperature of 1.083 K (272.067°C). It is strongly magnetic at room temperature. Hazards As with the other lanthanoids, gadolinium compounds are of low to moderate toxicity, although their toxicity has not been investigated in detail. Powder may react with water or moisture. The powder is highly flammable. Isotopes of Gadolinium Notable Isotopes 152 Gd [88 neutrons] Abundance: 0.20% Half life: 1.08 x 1014 years [ Alpha Decay ] Decay Energy: 2.205MeV Decays to 154 Sm. 148 Gd [90 neutrons] Abundance: 2.18% Stable with 90 neutrons 155 Gd [91 neutrons] Abundance: 14.80% Stable with 91 neutrons 156 Gd [92 neutrons] Abundance: 20.47% Stable with 92 neutrons 157 Gd [93 neutrons] Abundance: 15.65% Stable with 93 neutrons 158 Gd [94 neutrons] Abundance: 24.84% Stable with 94 neutrons 160 Gd [96 neutrons] Abundance: 21.86% Half life: 1.3 x 1021 years [ Double beta decay ] Decay Energy: 1.7MeV Decays to 160 Dy. Reactions of Gadolinium Reactions with water Gadolinium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form gadolinium hydroxide, Gd(OH)3, and hydrogen gas (H2). 2Gd(s) + 6H2O(g) 2Gd(OH)3(aq) + 3H2(g) Reactions with air Gadolinium metal burns readily in air to form Gadolinium(III) oxide. 4Gd(s) + 3O2(g) 2Gd2O3 Reactions with halogens Gadolinium metal reacts with all the halogens to form gadolinium(III) halides. 2Gd(s) + 3F2(g) 2GdF3(s) 2Gd(s) + 3Cl2(g) 2GdCl3(s) 2Gd(s) + 3Br2(g) 2GdBr3(s) 2Gd(s) + 3I2(g) 2GdI3(s) Reactions with acids Gadolinium metal dissolves readily in dilute sulphuric acid to form solutions containing the Gd(III) ion together with hydrogen gas. 2Gd(s) + 3H2SO4(aq) 2Gd3+(aq) + 3SO42-(aq) + 3H2(g) Dysprosium [Dy] CAS-ID: 7429-91-6 An: 66 N: 96 Am: 162.500 (1) g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 2840K (2567°C) Melting Point: 1680K (1407°C) Density: 8.540g/cm3 Discovery Information Who: Paul emile Lecoq de Boisbaudran When: 1886 Where: France Name Origin Greek: dysprositos (hard to get at). "Dysprosium" in different languages. Sources Dysprosium is never encountered as the free element. Usually found with erbium, holmium and other rare earths in some minerals (euxenite ((Y,Ca,Ce,U,Th)(Nb,Ta,Ti)2O6), fergusonite ((Ce,La,Nd)NbO4), gadolinite ((Ce,La,Nd,Y)2FeBe2Si2O10) and xenotime to name a few). Around 100 tons are produced annually. Primary mining occurs in the USA, Brazil, India, Sri Lanka and Australia. Abundance Universe: 0.002 ppm (by weight) Sun: 0.002 ppm (by weight) Carbonaceous meteorite: 0.28 ppm Earth's Crust: 6 ppm Seawater: Atlantic surface: 8 x 10-7 ppm Atlantic deep: 9.6 x 10-7 ppm Pacific surface: n/a ppm Pacific deep: n/a ppm Uses Dysprosium is used for manufacturing compact discs, and in conjunction with vanadium and other elements is used in making laser materials. As control-rods for nuclear reactors because it readily absorbs neutrons. History Dysprosium was first identified in Paris in 1886 by French chemist Paul Emile Lecoq de Boisbaudran. However, the element itself was not isolated in relatively pure form until after the development of ion exchange and metallographic reduction techniques in the 1950s. The name dysprosium is derived from the Greek "dysprositos"; "hard to obtain". Part of the difficulty lay in dysprosium being especially close in its behavior to the far more abundant yttrium, during many of the separation technologies that were used in the 19th century. This overshadowed the fact that dysprosium was the most abundant of the heavy lanthanoids. Notes It is soft enough to be cut with a knife, and can be machined without sparking if overheating is avoided. Dysprosium's characteristics can be greatly affected even by small amounts of impurities. It wasn't until the 1950s that the element was isolated in a relatively pure form. Dysprosium does not have any known biological properties. Hazards As with the other lanthanoids, dysprosium compounds are of low to moderate toxicity, although their toxicity has not been investigated in detail. Isotopes of Dysprosium Notable Isotopes 154 Dy [89 neutrons] Abundance: synthetic Half life: 3.0 x 106 years 156 Dy [90 neutrons] Abundance: 0.06% Stable with 90 neutrons 158 Dy [92 neutrons] Abundance: 0.10% Stable with 92 neutrons 160 Dy [94 neutrons] Abundance: 2.34% Stable with 94 neutrons 161 Dy [95 neutrons] Abundance: 18.91% Stable with 95 neutrons 162 Dy [96 neutrons] Abundance: 25.51% Stable with 96 neutrons 163 Dy [97 neutrons] Abundance: 24.90% Stable with 97 neutrons 164 Dy [98 neutrons] Abundance: 28.18% Stable with 98 neutrons Reactions of Dysprosium Reactions with water Dysprosium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form dysprosium hydroxide and hydrogen gas. 2Dy(s) + 6H2O(g) 2Dy(OH)3(aq) + 3H2(g) Reactions with air Dysprosium tarnishes slowly in air and burns readily to form dysprosium (III) oxide. 4Dy(s) + 3O2(g) 2Dy2O3(s) Reactions with halogens Dysprosium reacts with all the halogens to form dysprosium(III) halides. 2Dy(s) + 3F2(g) 2DyF3(s) 2Dy(s) + 3Cl2(g) 2DyCl3(s) 2Dy(s) + 3Br2(g) 2DyBr3(s) 2Dy(s) + 3I2(g) 2DyI3(s) Reactions with acids Dysprosium dissolves readily in dilute sulphuric acid to form solutions containing the yellow aquated Dy(III) ion together with hydrogen gas. 2Dy(s) + 3H2SO4(aq) 2Dy3+(aq) + 3SO42-(aq) + 3H2(g) Holmium [Ho] CAS-ID: 7440-60-0 An: 67 N: 98 Am: 164.93032 g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 2993K (2720°C) Melting Point: 1734K (1461°C) Density: 8.79g/cm3 Discovery Information Who: J.L. Soret When: 1878 Where: Switzerland Name Origin From Holmia, the Latin name for Stockholm. "Holmium" in different languages. Sources Occurs in gadolinite, monazie and other rare-earth minerals. Annual production is around 10 tons. Abundance Universe: 0.0005 ppm (by weight) Carbonaceous meteorite: 0.06 ppm Earth's Crust: 1.4 ppm Seawater: Atlantic surface: 2.4 x 10-7 ppm Atlantic deep: 2.9 x 10-7 ppm Pacific surface: 1.6 x 10-7 ppm Pacific deep: 1.6 x 10-7 ppm Uses As control-rods for nuclear reactors because it readily absorbs neutrons. Forms highly magnetic compounds when combined with yttrium. Holmium oxide is used as a yellow glass colouring. Its very high magnetic moment is suitable for use in yttrium-iron-garnet (YIG) and yttrium-lanthanum-fluoride (YLF) solid state lasers found in microwave equipment (which are in turn found in a variety of medical and dental settings). History Holmium (Holmia, Latin name for Stockholm) was discovered by Marc Delafontaine and Jacques-Louis Soret in 1878 who noticed the aberrant spectrographic absorption bands of the then-unknown element (they called it "Element X"). Later in 1878, Per Teodor Cleve independently discovered the element while he was working on erbia earth (erbium oxide). Using the method developed by Carl Gustaf Mosander, Cleve first removed all of the known contaminants from erbia. The result of that effort was two new materials, one brown and one green. He named the brown substance holmia (after the Latin name for Cleve's home town, Stockholm) and the green one thulia. Holmia was later found to be the holmium oxide and thulia was thulium oxide. Notes The element, as with other rare earth elements, appears to have a low acute toxic rating. Holmium plays no biological role in humans but may be able to stimulate metabolism. Isotopes of Holmium Notable Isotopes 165 Ho [98 neutrons] Abundance: 100% Stable with 98 neutrons Erbium [Er] CAS-ID: 7440-52-0 An: 68 N: 99 Am: 167.259 (3) g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 3141K (2868°C) Melting Point: 1770K (1497°C) Density: 9.066g/cm3 Discovery Information Who: Carl Mosander When: 1843 Where: Sweden Name Origin From Ytterby, Sweden. "Erbium" in different languages. Sources Found with other heavier rare earth metals, and in the minerals xenotime and euxenite ((Y,Ca,Ce,U,Th)(Nb,Ta,Ti)2O6). Around 500 tons are produced annually. Primary mining locations are in the USA, Brazil, India, Sri Lanka and Australia. Abundance Universe: 0.002 ppm (by weight) Sun: 0.001 ppm (by weight) Carbonaceous meteorite: 0.18 ppm Earth's Crust: 3.8 ppm Seawater: Atlantic surface: 5.9 x 10-7 ppm Atlantic deep: 8.6 x 10-7 ppm Pacific surface: n/a ppm Pacific deep: n/a ppm Uses For making photographic filters and as a neutron absorber. Erbium oxide is used in ceramics to obtain a pink glaze. When added to vanadium as an alloy erbium lowers hardness and improves workability. History Erbium (for Ytterby, a town in Sweden) was discovered by Carl Gustaf Mosander in 1843. Mosander separated "yttria" from the mineral gadolinite ((Ce,La,Nd,Y)2FeBe2Si2O10) into three fractions which he called yttria, erbia, and terbia. He named the new element after the town of Ytterby where large concentrations of yttria and erbium are located. Erbia and terbia, however, were confused at this time. After 1860, terbia was renamed erbia and after 1877 what had been known as erbia was renamed terbia. Fairly pure Er2O3 was independently isolated in 1905 by Georges Urbain and Charles James. Reasonably pure metal wasn't produced until 1934 when workers reduced the anhydrous chloride with potassium vapour. Notes A trivalent element, pure erbium metal is malleable, soft yet stable in air and does not oxidize as quickly as some other rare-earth metals. Its salts are rose-coloured and the element gives a characteristic sharp absorption spectra in visible light, ultraviolet, and near infrared. Otherwise it looks much like the other rare earths. Hazards Metallic erbium in dust form presents a fire and explosion hazard. Erbium is mostly dangerous in the working environment, due to the fact that damps and gasses can be inhaled with air. This can cause lung embolisms, especially during long-term exposure. Erbium can be a threat to the liver when it accumulates in the human body. Isotopes of Erbium Notable Isotopes 160 Er [92 neutrons] Abundance: synthetic Half life: 28.58 hours [ Electron Capture ] Decay Energy: 0.330MeV Decays to 162 160 Ho. Er [94 neutrons] Abundance: 0.14% Stable with 94 neutrons 164 Er [96 neutrons] Abundance: 1.61% Stable with 96 neutrons 165 Er [97 neutrons] Abundance: synthetic Half life: 10.36 hours [ Electron Capture ] Decay Energy: 0.376MeV Decays to 166 165 Ho. Er [98 neutrons] Abundance: 33.6% Stable with 98 neutrons 167 Er [99 neutrons] Abundance: 22.95% Stable with 99 neutrons 168 Er [100 neutrons] Abundance: 26.8% Stable with 100 neutrons 169 Er [101 neutrons] Abundance: synthetic Half life: 9.4 days [ beta- ] Decay Energy: 0.351MeV Decays to 170 169 Tm. Er [102 neutrons] Abundance: 14.9% Stable with 102 neutrons 171 Er [103 neutrons] Abundance: synthetic Half life: 7.516 hours [ beta- ] Decay Energy: 1.490MeV Decays to 172 171 Tm. Er [104 neutrons] Abundance: synthetic Half life: 49.3 hours [ beta- ] Decay Energy: 0.891MeV Decays to 172 Tm. Reactions of Erbium Reactions with water The erbium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form erbium hydroxide and hydrogen gas. 2Er(s) + 6H2O(g) 2Er(OH)3(aq) + 3H2(g) Reactions with air Erbium tarnishes slowly in air and burns readily to form erbium (III) oxide. 4Er(s) + 3O2(g) 2Er2O3(s) Reactions with halogens Erbium reacts with all the halogens to form erbium(III) halides. 2Er(s) + 3F2(g) 2ErF3(s) 2Er(s) + 3Cl2(g) 2ErCl3(s) 2Er(s) + 3Br2(g) 2ErBr3(s) 2Er(s) + 3I2(g) 2ErI3(s) Reactions with acids Erbium dissolves readily in dilute sulphuric acid to form solutions containing the yellow aquated Er(III) ion together with hydrogen gas. 2Er(s) + 3H2SO4(aq) 2Er3+(aq) + 3SO42-(aq) + 3H2(g) Thulium [Tm] CAS-ID: 7440-30-4 An: 69 N: 100 Am: 168.93421 g/mol Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 2223K (1950°C) Melting Point: 1818K (1545°C) Density: 9.32g/cm3 Discovery Information Who: Per Theodor Cleve When: 1879 Where: Sweden Name Origin From Thule ancient name of Scandinavia. "Thulium" in different languages. Sources Found with other rare earths in the minerals; monazite, gadolinite, euxenite ((Y,Ca,Ce,U,Th)(Nb,Ta,Ti)2O6), xenotime, and others. Primary producers are the USA, Brazil, India, Sri Lanka and Australia. Annual production is around 50 tons. Abundance Universe: 0.0001 ppm (by weight) Sun: 0.0002 ppm (by weight) Carbonaceous meteorite: 0.03 ppm Earth's Crust: 0.48 ppm Seawater: Atlantic surface: 1.3 x 10-7 ppm Atlantic deep: 1.6 x 10-7 ppm Pacific surface: 7 x 10-8 ppm Pacific deep: 3.3 x 10-7 ppm Uses None of thulium's compounds is commercially important mainly due to high production costs. Radioactive thulium is used to power portable x-ray machines, eliminating the need for electrical equipment. Thulium-doped calcium sulphate (CaSO4) has been used in personal radiation dosimeters because it can register, by its fluorescence, especially low levels. History Thulium was discovered by Swedish chemist Per Teodor Cleve in 1879 by looking for impurities in the oxides of other rare earth elements (this was the same method Carl Gustaf Mosander earlier used to discover some other rare earth elements). Cleve started by removing all of the known contaminants of erbia (Er2O3) and upon additional processing, obtained two new substances; one brown and one green. The brown substance turned out to be the oxide of the element holmium and was named holmia by Cleve and the green substance was the oxide of an unknown element. Cleve named the oxide thulia and its element thulium after Thule, Scandinavia. Thulium was so rare, that none of the early workers had enough of it to purify sufficiently to actually see the green colour; they had to be content with observing the strengthening of the two characteristic absorption bands, as erbium was progressively removed. The first researcher to obtain thulium nearly pure was the British expatriate working on a large scale at New Hampshire College in Durham NH: Charles James. In 1911, he reported his results, having used his discovered method of bromate fractional crystallization to do the purification. He famously needed 15,000 "operations" to establish that the material was homogeneous. Notes Thulium is the least abundant of the rare earth metals, is is and easy metal to work as it can be cut by a knife. Reserves of thulium are estimated to be about 10 5 tonnes. World production is about 50 tonnes per year as thulium oxide. Hazards Thulium has a low-to-moderate acute toxic rating and should be handled with care. Metallic thulium in dust form presents a fire and explosion hazard. Isotopes of Thulium Notable Isotopes 167 Tm [98 neutrons] Abundance: synthetic Half life: 9.25 days [ Electron Capture ] Decay Energy: 0.748MeV Decays to 168 167 Er. Tm [99 neutrons] Abundance: synthetic Half life: 93.1 days [ Electron Capture ] Decay Energy: 1.679MeV Decays to 169 168 Er. Tm [100 neutrons] Abundance: 100% Stable with 100 neutrons 170 Tm [101 neutrons] Abundance: synthetic Half life: 128.6 days [ beta- ] Decay Energy: 0.968MeV Decays to 171 170 Yb. Tm [102 neutrons] Abundance: synthetic Half life: 1.92 years [ beta- ] Decay Energy: 0.96MeV Decays to 171 Yb. Reactions of Thulium Reactions with water The silvery white metal thulium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form thulium hydroxide and hydrogen gas. 2Tm(s) + 6H2O(g) 2Tm(OH)3(aq) + 3H2(g) Reactions with air Thulium tarnishes slowly in air and burns readily to form thulium (III) oxide. 4Tm(s) + 3O2(g) 2Tm2O3(s) Reactions with halogens Thulium reacts with all the halogens to form thulium(III) halides. 2Tm(s) + 3F2(g) 2TmF3(s) 2Tm(s) + 3Cl2(g) 2TmCl3(s) 2Tm(s) + 3Br2(g) 2TmBr3(s) 2Tm(s) + 3I2(g) 2TmI3(s) Reactions with acids Thulium dissolves readily in dilute sulphuric acid to form solutions containing the pale green aquated Tm(III) ion together with hydrogen gas. 2Tm(s) + 3H2SO4(aq) Ytterbium [Yb] CAS-ID: 7440-64-4 An: 70 N: 103 Am: 173.04 (3) g/mol 2Tm3+(aq) + 3SO42-(aq) + 3H2(g) Group Name: Lanthanoids Block: f-block Period: 6 (lanthanoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 1469K (1196°C) Melting Point: 1097K (824°C) Density: 6.90g/cm3 Discovery Information Who: Jean de Marignac When: 1878 Where: Switzerland Name Origin From Ytterby, Sweden. "Ytterbium" in different languages. Sources Found in minerals such as yttria, monazite, gadolinite, and xenotime. Natural ytterbium is a mix of seven stable isotopes. Important producers are the USA, Canada, Greenland and Brazil. Annual production is around 50 tons. Abundance Universe: 0.002 ppm (by weight) Sun: 0.001 ppm (by weight) Carbonaceous meteorite: 0.18 ppm Earth's Crust: 3.3 ppm Seawater: Atlantic surface: 5 x 10-7 ppm Atlantic deep: 7.5 x 10-7 ppm Pacific surface: 3.7 x 10-7 ppm Pacific deep: 2.2 x 10-6 ppm Uses Used in metallurgical and chemical experiments. One ytterbium isotope has been used as a radiation source substitute for a portable Xray machine when electricity was not available. Ytterbium has a single absorption band at 985 nanometers, which is used to convert infrared energy into electricity in solar cells. History Ytterbium was discovered by the Swiss chemist Jean Charles Galissard de Marignac in 1878. Marignac found a new component in the earth then known as erbia and named it ytterbia (after Ytterby, the Swedish town where he found the new erbia component). He suspected that ytterbia was a compound of a new element he called ytterbium. In 1907, the French chemist Georges Urbain separated Marignac's ytterbia into two components, neoytterbia and lutecia. Neoytterbia would later become known as the element ytterbium and lutecia would later be known as the element lutetium. Auer von Welsbach independently isolated these elements from ytterbia at about the same time but called them aldebaranium and cassiopeium. Notes The chemical and physical properties of ytterbium could not be determined until 1953 when the first nearly pure ytterbium was produced Hazards Although it was thought that all ytterbium compounds were highly toxic, initial studies have shown that the danger is limited. Ytterbium compounds are known to cause skin and eye irritations, and may also be teratogenic. Metallic ytterbium dust poses a fire and explosion hazard. Isotopes of Ytterbium Notable Isotopes 166 Yb [96 neutrons] Abundance: synthetic Half life: 56.7 hours [ Electron Capture ] Decay Energy: 0.304MeV Decays to 168 166 Tm. Yb [98 neutrons] Abundance: 0.13% Stable with 98 neutrons 169 Yb [99 neutrons] Abundance: synthetic Half life: 32.026 days [ Electron Capture ] Decay Energy: 0.909MeV Decays to 170 169 Tm. Yb [100 neutrons] Abundance: 3.05% Stable with 100 neutrons 171 Yb [101 neutrons] Abundance: 14.3% Stable with 101 neutrons 172 Yb [102 neutrons] Abundance: 21.9% Stable with 102 neutrons 173 Yb [103 neutrons] Abundance: 16.12% Stable with 103 neutrons 174 Yb [104 neutrons] Abundance: 31.8% Stable with 104 neutrons 175 Yb [105 neutrons] Abundance: synthetic Half life: 4.185 days [ beta- ] Decay Energy: 0.470MeV Decays to 176 175 Lu. Yb [106 neutrons] Abundance: 12.7% Stable with 106 neutrons 177 Yb [107 neutrons] Abundance: synthetic Half life: 1.911 hours [ beta- ] Decay Energy: 1.399MeV Decays to 177 Lu. Reactions of Ytterbium Reactions with water Ytterbium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form ytterbium hydroxide and hydrogen gas. 2Yb(s) + 6H2O(g) 2Yb(OH)3(aq) + 3H2(g) Reactions with air Ytterbium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form ytterbium hydroxide and hydrogen gas. 4Yb + 3O2 2Yb2O3 Reactions with halogens Ytterbium metal reacts with all the halogens to form ytterbium(III) halides. 2Yb(s) + 3F2(g) 2YbF3(s) 2Yb(s) + 3Cl2(g) 2YbCl3(s) 2Yb(s) + 3Br2(g) 2YbBr3(s) 2Yb(s) + 3I2(g) 2YbI3(s) Reactions with acids Ytterbium metal dissolves readily in dilute sulphuric acid to form solutions containing the colourless aquated Yb(III) ion together with hydrogen gas. 2Yb(s) + 3H2SO4(aq) 2Yb3+(aq) + 3SO42-(aq) + 3H2(g)> Lutetium [Lu] CAS-ID: 7439-94-3 An: 71 N: 104 Am: 174.967 (1) g/mol Group No: 3 Group Name: Lanthanoids Block: d-block Period: 6 State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 3675K (3402°C) Melting Point: 1925K (1652°C) Superconducting temperature: 0.022K (-273.128°C) Density: 9.841g/cm3 Discovery Information Who: Georges Urbain When: 1907 Where: France Name Origin From Lutetia the ancient name of Paris. "Lutetium" in different languages. Sources Found with almost all other rare-earth metals but never by itself, lutetium is very difficult to separate from other elements. Consequently, it is also one of the most expensive metals, costing about six times as much as gold. The principal commercially viable ore of lutetium is the rare earth phosphate mineral monazite: (Ce, La, etc.)PO4 which contains 0.003% of the element. Pure lutetium metal has only relatively recently been isolated and is very difficult to prepare (thus it is one of the most rare and expensive of the rare earth metals). Primary mining locations are the USA, Brazil, India, Sri Lanka, Australia and Chine. Annual production is around 10 tons. Abundance Universe: 0.0001 ppm (by weight) Sun: 0.001 ppm (by weight) Carbonaceous meteorite: 0.03 ppm Earth's Crust: 0.51 ppm Seawater: Atlantic surface: 1.4 x 10-7 ppm Atlantic deep: 2 x 10-7 ppm Pacific surface: 6 x 10-8 ppm Pacific deep: 4.1 x 10-7 ppm Uses This element is very expensive to obtain in useful quantities and therefore it has very few commercial uses. Used in alloys and can be used as a catalyst in cracking, hydrogenation, polymerization and alkylation. A tiny amount of lutetium is added as a dopant to gadolinium gallium garnet (GGG), which is used in magnetic bubble memory devices. History Lutetium (Latin Lutetia meaning Paris) was independently discovered in 1907 by French scientist Georges Urbain and Austrian mineralogist Baron Carl Auer von Welsbach. Both men found lutetium as an impurity in the mineral ytterbia which was thought by Swiss chemist Jean Charles Galissard de Marignac (and most others) to consist entirely of the element ytterbium. The separation of lutetium from Marignac's ytterbium was first described by Urbain and the naming honor therefore went to him. He chose the names neoytterbium (new ytterbium) and lutecium for the new element but neoytterbium was eventually reverted back to ytterbium and in 1949 the spelling of element 71 was changed to lutetium. Welsbach proposed the names cassiopium for element 71 (after the constellation Cassiopeia) and albebaranium for the new name of ytterbium but these naming proposals where rejected (although many German scientists in the 1950s called the element 71 cassiopium). Notes Because of the difficulty in producing pure lutetium it is one of the most expensive metals, costing around six times as much per gram as gold. Hazards Like other rare-earth metals lutetium is regarded as having a low toxicity rating but it and especially its compounds should be handled with care nonetheless. Metal dust of this element is a fire and explosion hazard. Lutetium plays no biological role in the human body but is thought to help stimulate metabolism. Isotopes of Lutetium Notable Isotopes 173 Lu [102 neutrons] Abundance: synthetic Half life: 1.37 years [ Electron Capture ] Decay Energy: 0.671MeV Decays to 174 173 Yb. Lu [103 neutrons] Abundance: synthetic Half life: 3.31 years [ Electron Capture ] Decay Energy: 1.374MeV Decays to 175 174 Yb. Lu [104 neutrons] Abundance: 97.41% Stable with 104 neutrons 176 Lu [105 neutrons] Abundance: 2.59% Half life: 3.78 x 1010 years [ beta- ] Decay Energy: 1.193MeV Decays to 176 Hf. Reactions of Lutetium Reactions with water Lutetium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form lutetium hydroxide and hydrogen gas. 2Lu(s) + 6H2O(g) 2Lu(OH)3(aq) + 3H2(g) Reactions with air Lutetium metal tarnishes slowly in air and burns readily to form lutetium (III) oxide. 4Lu + 3O2 2Lu2O3 Reactions with halogens Lutetium metal reacts with all the halogens to form lutetium(III) halides. 2Lu(s) + 3F2(g) 2LuF3(s) 2Lu(s) + 3Cl2(g) 2LuCl3(s) 2Lu(s) + 3Br2(g) 2LuBr3(s) 2Lu(s) + 3I2(g) 2LuI3(s) Reactions with acids Lutetium dissolves readily in dilute sulphuric acid to form solutions containing the colourless aquated Lu(III) ion together with hydrogen gas. 2Lu(s) + 3H2SO4(aq) Thorium [Th] CAS-ID: 7440-29-1 An: 90 N: 142 Am: 232.0381 g/mol Group Name: Actinoid 2Lu3+(aq) + SO42-(aq) + 3H2(g) Block: f-block Period: 7 (actinoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 5093K (4820°C) Melting Point: 2115K (1842°C) Superconducting temperature: 1.38K (-271.77°C) Density: 11.7g/cm3 Discovery Information Who: Jons Berzelius When: 1828 Where: Sweden Name Origin From the Scandinavian god Thor. "Thorium" in different languages. Sources Found in various minerals like monazite and thorite ((Th,U)SiO4). Thorium is found in small amounts in most rocks and soils (about 6ppm), where it is three times more abundant than uranium and about as common as lead. Primary producers are the USA, Brazil, India, Sri Lanka, Madagascar, Russia and Australia. Annual production is around 31 thousand tons. Abundance Universe: 0.0004 ppm (by weight) Sun: 0.0003 ppm (by weight) Carbonaceous meteorite: 0.04 ppm Earth's Crust: 12 ppm Seawater: 9.2 ppm Uses Used in making strong alloys, ultraviolet photoelectric cells, mantles in portable gas lights and for coating tungsten wire in electronic equipment. Bombarded with neutrons make uranium-233, a nuclear fuel. Thorium dioxide (ThO2) is used in producing high-temperature laboratory crucibles. When added to glass it helps create glasses of a high refractive index and with low dispersion. Consequently, they find application in high-quality lenses for cameras and scientific instruments. History M. T. Esmark found a black mineral on Lovoy Island, Norway and gave a sample to Professor Jens Esmark, a noted mineralogist who was not able to identify it so he sent a sample to the Swedish chemist Jons Jakob Berzelius for examination in 1828. Berzelius analysed it and named it after Thor, the Norse god of thunder. The metal had virtually no uses until the invention of the gas mantle in 1885. The crystal bar process (or Iodide process) was discovered by Anton Eduard van Arkel and Jan Hendrik de Boer in 1925 to produce high-purity metallic thorium. The name ionium was given early in the study of radioactive elements to the 230Th isotope produced in the decay chain of 238U before it was realized that ionium and thorium were chemically identical. The symbol Io was used for this supposed element. Notes Thorium dioxide (ThO2), also called thoria, has one of the highest melting points of all oxides (3300°C). Isotopes of Thorium Notes Thorium has no stable isotopes. Notable Isotopes 228 Th [138 neutrons] Abundance: Synthetic Half life: 1.9116 years [ Alpha Decay ] Decay Energy: 5.520MeV Decays to 229 Ra. 224 Th [139 neutrons] Abundance: Synthetic Half life: 7340 years [ Alpha Decay ] Decay Energy: 5.168MeV Decays to 230 Ra. 225 Th [140 neutrons] Abundance: Synthetic Half life: 75380 years [ Alpha Decay ] Decay Energy: 4.770MeV Decays to 232 Ra. 226 Th [142 neutrons] Abundance: 100% Half life: 1.4505 x 1010years [ Alpha Decay ] Decay Energy: 4.083MeV Decays to Ra. 228 Protactinium [Pa] CAS-ID: 7440-13-3 An: 91 N: 122 Am: 231.03588 g/mol Group Name: Actinoid Block: f-block Period: 7 (actinoid) State: solid at 298 K Colour: silvery metallic Classification: Metallic Boiling Point: 4300K (4300°C) Melting Point: 1841K (1568°C) Superconducting temperature: 1.4K (-271.7°C) Density: 15.37g/cm3 Discovery Information Who: Fredrich Soddy, John Cranston, Otto Hahn, Lise Meitner When: 1917 Where: England/France Name Origin protos (first); its is the parent of actinium, which is formed by radioactive decay. "Protactinium" in different languages. Sources Does not occur in nature. Found among fission products of uranium, thorium, and plutonium. Protactinium occurs in pitchblende to the extent of about 1 part 231Pa to 10 million of ore. Some ores from the Democratic Republic of the Congo have about 3 ppm. Abundance Earth's Crust: 1 x 10-8 ppm Seawater: 2 x 10-11 ppm Uses Because of its scarcity, high radioactivity and toxicity, there are currently no uses for protactinium outside of basic scientific research. History An element between thorium and uranium was predicted to exist by Mendeleev in 1871. In 1900 William Crookes isolated protactinium as a radioactive material from uranium which he could not identify Protactinium was first identified in 1913, when Kasimir Fajans and O. H. Gohring encountered short-lived isotope 234m-Pa, with a half-life of about 1.17 minutes, during their studies of the decay chain of 238-U. They gave the new element the name Brevium (Latin brevis, brief, short); the name was changed to Protoactinium in 1918 when two groups of scientists (Otto Hahn and Lise Meitner of Germany and Frederick Soddy and John Cranston of the UK) independently discovered 231-Pa, and shortened to Protactinium in 1949. Aristid V. Grosse prepared 2 mg of Pa2O5 in 1927, and later on managed to isolate Protactinium for the first time in 1934 from 0.1 mg of Pa2O5, first converting the oxide to an iodide and then cracking it in a high vacuum by an electrically heated filament. In 1961, the United Kingdom Atomic Energy Authority was able to produce 125 g of 99.9% pure protactinium, processing 60 tons of waste material in a 12-stage process and spending 500,000 USD; this was the world's only supply of the element for many years to come, and it is reported that the metal was sold to laboratories for a cost of 2,800 USD / g in the following years. Notes It is superconductive at temperatures below 1.4 K (-271.74°C). 29 radioisotopes of protactinium have been characterized, with the most stable being 231 Pa with a half life of 32760 years, 233Pa with a half-life of 26.967 days, and 230Pa with a half-life of 17.4 days. All of the remaining radioactive isotopes have half-lives that are less than 1.6 days, and the majority of these have half lifes that are less than 1.8 seconds. Hazards Protactinium is both toxic and highly radioactive. Isotopes of Protactinium Notes Protactinium has no stable isotopes. Notable Isotopes 230 Pa [139 neutrons] Abundance: synthetic Half life: 17.4 days [ Electron Capture ] Decay Energy: 1.310MeV Decays to 230 Th. Half life: 17.4 days [ beta- ] Decay Energy: 0.563MeV Decays to 231 U. 230 Pa [140 neutrons] Abundance: synthetic Half life: 32760 years [ Alpha Decay ] Decay Energy: 5.149MeV Decays to 233 Ac. 227 Pa [142 neutrons] Abundance: synthetic Half life: 26.967 days [ beta- ] Decay Energy: 0.571MeV Decays to U. 233 Uranium [U] CAS-ID: 7440-61-1 An: 92 N: 146 Am: 238.02891 (3) g/mol Group Name: Actinoid Block: f-block Period: 7 (actinoid) State: solid at 298 K Colour: metallic grey Classification: Metallic Boiling Point: 4200K (3927°C) Melting Point: 1405.3K (1132.2°C) Superconducting temperature: 0.2K (-272.9°C) Density: 19.1g/cm3 Discovery Information Who: Martin Klaproth When: 1789 Where: Germany Name Origin From planet Uranus. "Uranium" in different languages. Sources Occurs in many rocks, but in large amounts only in such minerals as pitchblende and carnotite (K2(UO2)2(VO4)2 - 3H2O). Annual production is around 35 thousand tons. Abundance Universe: 0.0002 ppm (by weight) Sun: 0.001 ppm (by weight) Carbonaceous meteorite: 0.010 ppm Earth's Crust: 1.8 ppm Seawater: 3.13 x 10-3 ppm Human: 1 ppb by weight 0.03 ppb by atoms Uses For many centuries it was used as a pigment for glass. Now it is used as a fuel in nuclear reactors and in nuclear bombs. Depleted Uranium ( 238U) is used in casings of armour piercing artillery shells, armour plating on tanks and as ballast in the wings of some large aircraft. History The use of uranium in its natural oxide form dates back to at least the year AD79, when it was used to add a yellow colour to ceramic glazes. Yellow glass with 1% uranium oxide was found in a Roman villa on Cape Posillipo in the Bay of Naples, Italy by R. T. Gunther of the University of Oxford in 1912. Starting in the late Middle Ages, pitchblende was extracted from the Habsburg silver mines in Joachimsthal, Bohemia (now Jachymov in the Czech Republic) and was used as a colouring agent in the local glassmaking industry. In the early 19th century, the world's only known source of uranium ores were these old mines. The discovery of the element is credited to the German chemist Martin Heinrich Klaproth. While he was working in his experimental laboratory in Berlin in 1789, Klaproth was able to precipitate a yellow compound (likely sodium diuranate) by dissolving pitchblende in nitric acid and neutralizing the solution with sodium hydroxide. Klaproth mistakenly assumed the yellow substance was the oxide of a yetundiscovered element and heated it with charcoal to obtain a black powder, which he thought was the newly discovered metal itself (in fact, that powder was an oxide of uranium). He named the newly discovered element after the planet Uranus, which had been discovered eight years earlier by William Herschel. In 1841, Eugene-Melchior Peligot, who was Professor of Analytical Chemistry at the Conservatoire des arts et metiers (Central School of Arts and Manufactures) in Paris, isolated the first sample of uranium metal by heating uranium tetrachloride with potassium. Uranium was not seen as being particularly dangerous during much of the 19th century, leading to the development of various uses for the element. One such use for the oxide was the aforementioned but no longer secret colouring of pottery and glass. Antoine Becquerel discovered radioactivity by using uranium in 1896. Becquerel made the discovery in Paris by leaving a sample of uranium on top of an unexposed photographic plate in a drawer and noting that the plate had become 'fogged'. He determined that a form of invisible light or rays emitted by uranium had exposed the plate. Notes Uranium metal has very high density, 65% more dense than lead, but slightly less dense than gold. 70% of the world's known Uranium is located in Australia. The Australian government is currently advocating an expansion of uranium mining, although issues with state governments and indigenous interests complicate the issue. Hazards Potential occupational carcinogen (lung cancer). All isotopes and compounds of uranium are very toxic, teratogenic and radioactive. Finely-divided uranium metal presents a fire hazard because uranium is pyrophoric, so small grains will ignite spontaneously in air at room temperature. Isotopes of Uranium Notes Uranium has no stable isotopes. Notable Isotopes U [140 neutrons] 232 Abundance: synthetic Half life: 68.9 years Decay Energy: 5.414MeV Decays to 228 Th. U [141 neutrons] 233 Abundance: synthetic Half life: 159200 years Decay Energy: 4.909MeV Decays to 229 Th. U [142 neutrons] 234 Abundance: 0.006% Half life: 245500 years Decay Energy: 4.859MeV Decays to 230 Th. U [143 neutrons] 235 Abundance: 0.72% Half life: 7.038 x 108 years Decay Energy: 4.679MeV Decays to 231 Th. U is unique in its ability to cause a rapidly expanding fission chain reaction, i.e., it is fissile. In fact, U-235 is the only fissile isotope found in nature. It was discovered in 1935 by Arthur Jeffrey Dempster. A uranium nucleus that absorbs a neutron splits into two lighter nuclei; this is called nuclear fission. It releases either two or three neutrons which continue the reaction. In nuclear reactors, the reaction is slowed down by the addition of control rods which are made of elements such as boron, cadmium, and hafnium which can absorb a large number of neutrons. In nuclear bombs, the reaction is uncontrolled and the large amount of energy released creates a nuclear explosion. 235 U [144 neutrons] 236 Abundance: synthetic Half life: 2.342 x 107 years Decay Energy: 4.572MeV Decays to 232 Th. U [146 neutrons] 238 Abundance: 99.275% Half life: 4.468 x 109 years Decay Energy: 4.260MeV Decays to 234 Th. Neptunium [Np] CAS-ID: 7439-99-8 An: 93 N: 144 Am: [237] g/mol Group Name: Actinoid Block: f-block Period: 7 (actinoid) State: solid at 298 K Colour: silvery metallic Classification: Metallic Boiling Point: 4273K (4000°C) Melting Point: 910K (637°C) Density: 20.2g/cm3 Discovery Information Who: E.M. McMillan, P.H. Abelson When: 1940 Where: United States Name Origin From planet Neptune. "Neptunium" in different languages. Sources Produced by bombarding uranium with slow neutrons. Neptunium is also found in trace amounts in uranium ores. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a component in neutron detection equipment. Uses Used in neutron detection instruments. Np is irradiated with neutrons to create spacecraft and military applications. 237 Pu, a rare and valuable isotope for 238 History Neptunium (named for the planet Neptune, the next planet out from Uranus, after which uranium was named) was first discovered by Edwin McMillan and Philip H. Abelson in 1940. Initially predicted by Walter Russell's "spiral" organization of the periodic table, it was found at the Berkeley Radiation Laboratory of the University of California, Berkeley where the team produced the neptunium isotope 239Np (2.4 day half-life) by bombarding uranium with slow moving neutrons. It was the first transuranium element produced synthetically and the first actinoids series transuranium element discovered. Notes It was the first transuranium element produced synthetically and the first actinoid series transuranium element discovered. 19 neptunium radioisotopes have been characterized, with the most stable being 237Np with a half-life of 2.14 million years, 236Np with a half-life of 154,000 years, and 235Np with a half-life of 396.1 days. All of the remaining radioactive isotopes have half-lives that are less than 4.5 days, and the majority of these have half-lives that are less than 50 minutes. Isotopes of Neptunium Notes Neptunium has no stable isotopes. Notable Isotopes 235 Np [142 neutrons] Abundance: synthetic Half life: 396.1 days [ Alpha Decay ] Decay Energy: 5.192MeV Decays to 231 Pa. Half life: 396.1 days [ Electron Capture ] Decay Energy: 0.124MeV Decays to 236 U. 235 Np [143 neutrons] Abundance: synthetic Half life: 154 x 103 years [ Electron Capture ] Decay Energy: 0.940MeV Decays to U. 236 Half life: 154 x 103 years [ beta- ] Decay Energy: 0.940MeV Decays to 236 Pu. Half life: 154 x 103 years [ Alpha Decay ] Decay Energy: 5.020MeV Decays to 237 232 Pa. Np [144 neutrons] Abundance: synthetic Half life: 2.144 x 106 years Decay Energy: 4.959MeV Decays to 232 Pa. Plutonium [Pu] CAS-ID: 7440-07-5 An: 94 N: 150 Am: [244] g/mol Group Name: Actinoid Block: f-block Period: 7 (actinoid) State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 3503K (3230°C) Melting Point: 912.5K (639.4°C) Density: 19.816g/cm3 Discovery Information Who: G.T.Seaborg, J.W.Kennedy, E.M.McMillan, A.C.Wahl When: 1940 Where: United States Name Origin From planet Pluto. "Plutonium" in different languages. Sources Almost all plutonium is manufactured synthetically, extremely tiny trace amounts are found naturally in uranium ores. Most plutonium is made synthetically by bombarding uranium with neutrons. Annual production is around 20 tons, it is thought that world reserves are around 500 tons. Uses Used in bombs and reactors. Complete detonation of plutonium will produce an explosion equivalent to 20 kilotons of Trinitrotoluene (TNT) per kilogram (of plutonium). History The production of plutonium and neptunium by bombarding uranium-238 with neutrons was predicted in 1940 by two teams working independently: Edwin M. McMillan and Philip Abelson at Berkeley Radiation Laboratory at the University of California, Berkeley and by Egon Bretscher and Norman Feather at the Cavendish Laboratory at University of Cambridge. Coincidentally both teams proposed the same names to follow on from uranium, like the sequence of the outer planets. Plutonium was first produced and isolated on February 23, 1941 by Dr. Glenn T. Seaborg, Dr. Michael Cefola, Edwin M. McMillan, J. W. Kennedy, and A. C. Wahl by deuteron bombardment of uranium in the 60-inch cyclotron at Berkeley. The discovery was kept secret due to the war. It was named after Pluto, having been discovered directly after neptunium (which itself was one higher on the periodic table than uranium), by analogy to solar system planet order as Pluto was considered to be a planet at the time (though technically it should have been "plutium", Seaborg said that he did not think it sounded as good as "plutonium"). Seaborg chose the letters "Pu" as a joke, which passed without notice into the periodic table. Originally, Seaborg and others thought about naming the element "ultinium" or "extremium" because they believed at the time that they had found the last possible element on the periodic table. Chemists at the University of Chicago began to study the newly manufactured radioactive element. The George Herbert Jones Laboratory at the university was the site where, for the first time, a trace quantity of this new element was isolated and measured in September 1942. This procedure enabled chemists to determine the new element's atomic weight. Room 405 of the building was named a National Historic Landmark in May 1967. During the Manhattan Project, the first production reactor was built at the Oak Ridge, Tennessee site that later became Oak Ridge National Laboratory. Later, large reactors were set up in Hanford, Washington, for the production of plutonium, which was used in the first atomic bomb used at the "Trinity" test at White Sands, New Mexico in July 1945. Plutonium was also used in the "Fat Man" bomb dropped on Nagasaki, Japan in August 1945. The "Little Boy" bomb dropped on Hiroshima utilized uranium-235, not plutonium. During the initial years after the discovery of plutonium, when its biological and physical properties were very poorly understood, a series of human radiation experiments were performed by the U.S. government and by private organizations acting on its behalf. During and after the end of World War II, scientists working on the Manhattan Project and other nuclear weapons research projects conducted studies of the effects of plutonium on laboratory animals and human subjects. In the case of human subjects, this involved injecting solutions containing (typically) five micrograms of plutonium into hospital patients thought to be either terminally ill, or to have a life expectancy of less than ten years either due to age or chronic disease condition. These eighteen injections were made without the informed consent of those patients and were not done with the belief that the injections would heal their conditions; rather, they were used to develop diagnostic tools for determining the uptake of plutonium in the body for use in developing safety standards for people working with plutonium during the course of developing nuclear weapons. Notes The heat given off by alpha particle emission makes plutonium warm to the touch in reasonable quantities; larger amounts can boil water. All isotopes and compounds of plutonium are toxic and radioactive. Hazards Plutonium is radioactive. When taken in by mouth, plutonium is less poisonous (except for risk of causing cancer) than several common substances including caffeine, acetaminophen, some vitamins, pseudoephedrine, and any number of plants and fungi. It is perhaps somewhat more poisonous than pure ethanol (C 2H5OH), but less so than tobacco; and many illegal drugs. From a purely chemical standpoint, it is about as poisonous as lead and other heavy metals. Isotopes of Plutonium Notes Plutonium has no stable isotopes. Notable Isotopes 238 Pu [144 neutrons] Abundance: synthetic Half life: 88 years Decay Energy: ?MeV Decays to ?. Half life: 88 years [ Alpha Decay ] Decay Energy: 5.5MeV Decays to 239 U. 234 Pu [145 neutrons] Abundance: trace Half life: 24.1 x 103 years Decay Energy: ?MeV Decays to ?. Half life: 24.1 x 103 years [ Alpha Decay ] Decay Energy: 5.245MeV Decays to 240 U. 235 Pu [146 neutrons] Abundance: synthetic Half life: 6.5 x 103 years Decay Energy: ?MeV Decays to ?. Half life: 6.5 x 103 years Decay Energy: 0.005MeV Decays to 241 Am. 240 Pu [147 neutrons] Abundance: synthetic Half life: 14 years [ Alpha Decay ] Decay Energy: 4.9MeV Decays to U. 237 Half life: 14 years Decay Energy: ?MeV 242 Pu [148 neutrons] Abundance: synthetic Half life: 3.73x 105 years Decay Energy: ?MeV Decays to ?. Half life: 3.73x 105 years [ Alpha Decay ] Decay Energy: 4.984MeV Decays to 244 U. 238 Pu [150 neutrons] Abundance: synthetic Half life: 8.08 x 107 years [ Alpha Decay ] Decay Energy: 4.66MeV Decays to U. 240 Half life: 8.08 x 107 years Decay Energy: ?MeV Decays to ?. Americium [Am] CAS-ID: 7440-35-9 An: 95 N: 148 Am: [243] g/mol Group Name: Actinoid Block: f-block Period: 7 (actinoid State: solid at 298 K Colour: silvery white Classification: Metallic Boiling Point: 2880K (2607°C) Melting Point: 1449K (1176°C) Superconducting temperature: 0.6K (-272.5°C) Density: 12g/cm3 Discovery Information Who: G. T. Seaborg, R. A. James, L. O. Morgan, A. Ghiorso When: 1945 Where: United States Name Origin From America by analogy with europium. "Americium" in different languages. Sources Produced by bombarding plutonium with neutrons. Uses Americium-241 is currently used in smoke detectors. The element has also been employed to gauge glass thickness to help create flat glass. History Americium was first isolated by Glenn T. Seaborg, Leon O. Morgan, Ralph A. James, and Albert Ghiorso in late 1944 at the wartime Metallurgical Laboratory at the University of Chicago (now known as Argonne National Laboratory). The team created the isotope 241Am by subjecting 239Pu to successive neutron capture reactions in a nuclear reactor. This created 240Pu and then 241Pu which in turn decayed into 241Am via beta decay. The discovery of americium and curium was first announced informally on a children's quiz show in 1945. Notes Pure americium has a silvery and white lustre. At room temperatures it slowly tarnishes in dry air. It is more silvery than plutonium or neptunium and apparently more malleable than neptunium or uranium. Alpha emission from 241Am is approximately three times that of radium. 18 radioisotopes of americium have been characterized, with the most stable being 243 Am with a half-life of 7370 years, and 241Am with a half-life of 432.2 years. All of the remaining radioactive isotopes have half-lives that are less than 51 hours, and the majority of these have half-lives that are less than 100 minutes. Hazards Americium is radioactive. Alpha emission from 241Am is approximately three times that of radium. Gram quantities of 241Am emit intense gamma rays which creates a serious exposure problem for anyone handling the element. Isotopes of Americium Notable Isotopes Am [146 neutrons] 241 Abundance: synthetic Half life: 432.2 years Decay Energy: ?MeV Decays to ?. Half life: 432.2 years [ Alpha Decay ] Decay Energy: 5.638MeV Decays to 242m 237 Np. Am [147 neutrons] Abundance: synthetic Half life: 141 years Decay Energy: 0.049MeV Decays to ?. Half life: 141 years [ Alpha Decay ] Decay Energy: 5.637MeV Decays to 238 Np. Half life: 141 years Decay Energy: ?MeV Decays to ?. Am [148 neutrons] 243 Abundance: synthetic Half life: 7370 years Decay Energy: ?MeV Decays to ?. Half life: 7370 years [ Alpha Decay ] Decay Energy: 5.438MeV Decays to 239 Np. Curium [Cm] CAS-ID: 7440-51-9 An: 96 N: 151 Am: [247] g/mol Group Name: Actinoid Block: f-block Period: 7 (actinoid) State: solid at 298 K Colour: silver Classification: Metallic Boiling Point: 3383K (3110°C) Melting Point: 1613K (1340°C) Density: 13.51g/cm3 Discovery Information Who: G.T.Seaborg, R.A.James, A. Ghiorso When: 1944 Where: United States Name Origin In honour of Pierre and Marie Curie. "Curium" in different languages. Sources Made by bombarding plutonium with helium ions. Curium was made in elemental form for the first time in 1951. Uses As curium is only available in extremely limited quantities, it has few uses, however, it was used on a Mars mission as an alpha particle source for the Alpha Proton X-Ray Spectrometer. History Curium was first synthesized at the University of California, Berkeley by Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944. The team named the new element after Marie Curie and her husband Pierre who are famous for discovering radium and for their work in radioactivity. It was chemically identified at the Metallurgical Laboratory (now Argonne National Laboratory) at the University of Chicago. It was actually the third transuranium element to be discovered even though it is the second in the series. Curium-242 (half-life 163 days) and one free neutron were made by bombarding alpha particles onto a plutonium-239 target in the 60-inch cyclotron at Berkeley. Louis Werner and Isadore Perlman created a visible sample of curium-242 hydroxide at the University of California in 1947 by bombarding americium-241 with neutrons. Curium was made in its elemental form in 1951 for the first time. Notes The isotope curium-248 has been synthesized only in milligram quantities, but curium242 and curium-244 are made in multigram amounts, which allows for the determination of some of the element's properties. Curium-242 can generate up to 120 watts of thermal energy per gram (W/g); its very short half-life though makes it undesirable as a power source for long-term use. 19 radioisotopes of curium have been characterized, with the most stable being Cm247 with a half-life of 1.56 x 107 years, Cm-248 with a half-life of 3.40 x 105 years, Cm-250 with a half-life of 9000 years, and Cm-245 with a half-life of 8500 years. All of the remaining radioactive isotopes have half-lifes that are less than 30 years, and the majority of these have half lifes that are less than 33 days. So radioactive it glows in the dark. Hazards Curium bio-accumulates in bone tissue where its radiation destroys bone marrow and thus stops red blood cell creation. Isotopes of Curium Notes Curium has no stable isotopes. Notable Isotopes 242 Cm [146 neutrons] Abundance: synthetic Half life: 160 days Decay Energy: ?MeV Half life: 160 days [ Alpha Decay ] Decay Energy: 6.1MeV Decays to 243 238 Pu. Cm [147 neutrons] Abundance: synthetic Half life: 29.1 years [ Alpha Decay ] Decay Energy: 6.169MeV Decays to 239 Pu. Half life: 29.1 years [ Electron Capture ] Decay Energy: 0.009?MeV Decays to 244 Am?. 243 Cm [148 neutrons] Abundance: synthetic Half life: 18.1 years Decay Energy: ?MeV Decays to ?. Half life: 18.1 years [ Alpha Decay ] Decay Energy: 5.902MeV Decays to 245 240 Pu. Cm [149 neutrons] Abundance: synthetic Half life: 8500 years Decay Energy: ?MeV Decays to ?. Half life: 8500 years [ Alpha Decay ] Decay Energy: 5.623MeV Decays to 246 241 Pu. Cm [150 neutrons] Abundance: synthetic Half life: 4730 years [ Alpha Decay ] Decay Energy: 5.475MeV Decays to 242 Pu. Half life: 4730 years Decay Energy: ?MeV Decays to ?. 247 Cm [151 neutrons] Abundance: synthetic Half life: 1.56 x 107 years [ Alpha Decay ] Decay Energy: 5.353MeV Decays to 248 243 Pu. Cm [152 neutrons] Abundance: synthetic Half life: 3.40 x 105 years [ Alpha Decay ] Decay Energy: 5.162MeV Decays to 244 Pu. Half life: 3.40 x 105 years Decay Energy: ?MeV Decays to ?. 250 Cm [154 neutrons] Abundance: synthetic Half life: 9000 years Decay Energy: ?MeV Decays to ?. Half life: 9000 years [ Alpha Decay ] Decay Energy: 5.169MeV Decays to 246 Pu. Half life: 9000 years [ beta- ] Decay Energy: 0.037MeV Decays to 250 Bk. Berkelium [Bk] CAS-ID: 7440-40-6 An: 97 N: 150 Am: [247] g/mol Group Name: Actinoid Block: f-block Period: 7 (actinoid) State: solid Colour: unknown, but probably metallic and silvery white or grey in appearance Classification: Metallic Boiling Point: unknown Melting Point: 1259K (986°C) Density: (alpha) 14.78g/cm3 Density: (beta) 13.25g/cm3 Discovery Information Who: G.T.Seaborg, S.G.Tompson, A. Ghiorso When: 1949 Where: United States Name Origin After Berkeley the home town of the University of California. "Berkelium" in different languages. Sources Some compounds have been made and studied. Made by bombarding americium with alpha particles. Uses Berkelium has no known uses outside of basic research. History Berkelium was first synthesized by Glenn T. Seaborg, Albert Ghiorso, Stanley G. Thompson, and Kenneth Street, Jr at the University of California, Berkeley in December 1949. The team used a cyclotron to bombard a milligram-sized target of 241 Am with alpha particles to produce 243Bk (half-life 4.5 hours) and two free neutrons. One of the longest lived isotopes of the element, 249Bk (half-life 330 days), was later synthesized by subjecting a 244Cm target with an intense beam of neutrons. Notes Weighable amounts of 249Bk (half-life 314 days) make it possible to determine some of its properties using macroscopic quantities. Berkelium is a radioactive rare earth metal. It is named after the University of California at Berkeley (USA). Apparently, berkelium tends to accumulate in the skeletal system. It is of no commercial importance and only a few of its compounds are known. Hazards Berkelium is radioactive. Like other actinoids, berkelium bio-accumulates in skeletal tissue. Isotopes of Berkelium Notes Berkelium has no stable isotopes. Notable Isotopes Bk [148 neutrons] 245 Abundance: synthetic Half life: 4.94 days [ Electron Capture ] Decay Energy: 0.810MeV Decays to 245 Cm. Half life: 4.94 days [ Alpha Decay ] Decay Energy: 6.455MeV Decays to Am. 241 Bk [149 neutrons] 246 Abundance: synthetic Half life: 1.8 days [ Alpha Decay ] Decay Energy: 6.070MeV Decays to Am. 242 Half life: 1.8 days [ Electron Capture ] Decay Energy: 1.350MeV Decays to 246 Cm. Bk [150 neutrons] 247 Abundance: synthetic Half life: 1380 years [ Alpha Decay ] Decay Energy: 5.889MeV Decays to Am. 243 Bk [151 neutrons] 248 Abundance: synthetic Half life: 9+ years [ Alpha Decay ] Decay Energy: 5.803MeV Decays to Am. 244 Bk [152 neutrons] 249 Abundance: synthetic Half life: 320 days [ Alpha Decay ] Decay Energy: 5.526MeV Decays to Am. 245 Half life: 320 days Decay Energy: ?MeV Decays to ?. Half life: 320 days [ beta- ] Decay Energy: 0.125MeV Decays to 249 Cf. Isotopes of Californium Notes Californium has no stable isotopes. Notable Isotopes 248 Cf [150 neutrons] Abundance: synthetic Half life: 333.5 days Decay Energy: ?MeV Decays to ?. Half life: 333.5 days [ Alpha Decay ] Decay Energy: 6.361MeV Decays to 249 244 Cm. Cf [151 neutrons] Abundance: synthetic Half life: 351 years Decay Energy: ?MeV Decays to ?. Half life: 351 years [ Alpha Decay ] Decay Energy: 6.295MeV Decays to 250 245 Cm. Cf [152 neutrons] Abundance: synthetic Half life: 13.08 years [ Alpha Decay ] Decay Energy: 6.128MeV Decays to 246 Cm. Half life: 13.08 years Decay Energy: ?MeV Decays to ?. 251 Cf [153 neutrons] Abundance: synthetic Half life: 898 years [ Alpha Decay ] Decay Energy: 6.176MeV Decays to 252 247 Cm. Cf [154 neutrons] Abundance: synthetic Half life: 2.645 years [ Alpha Decay ] Decay Energy: 6.217MeV Decays to 248 Cm. Half life: 2.645 years Decay Energy: ?MeV Decays to ?. 253 Cf [155 neutrons] Abundance: synthetic Half life: 17.81 days [ beta- ] Decay Energy: 0.285MeV Decays to 253 Es. Half life: 17.81 days [ Alpha Decay ] Decay Energy: 6.124MeV Decays to 254 249 Cm. Cf [156 neutrons] Abundance: synthetic Half life: 60.5 days Decay Energy: ?MeV Decays to ?. Half life: 60.5 days [ Alpha Decay ] Decay Energy: 5.926MeV Decays to 250 Cm. Isotopes of Einsteinium Notes Einsteinium has no stable isotopes. Notable Isotopes 252 Es [153 neutrons] Abundance: synthetic Half life: 471.7 days [ Alpha Decay ] Decay Energy: 6.760MeV Decays to 248 Bk. Half life: 471.7 days [ Electron Capture ] Decay Energy: 1.260MeV Decays to 252 Cf. Half life: 471.7 days [ beta- ] Decay Energy: 0.480MeV Decays to 253 Fm. 252 Es [154 neutrons] Abundance: synthetic Half life: 20.47 Decay Energy: ?MeV Decays to ?. Half life: 20.47 [ Alpha Decay ] Decay Energy: 6.739MeV Decays to 254 249 Bk. Es [155 neutrons] Abundance: synthetic Half life: 275.7 days [ Electron Capture ] Decay Energy: 0.654MeV Decays to 254 Cf. Half life: 275.7 days [ beta- ] Decay Energy: 1.090MeV Decays to Fm. 254 Half life: 275.7 days [ Alpha Decay ] Decay Energy: 6.628MeV Decays to 255 250 Bk. Es [156 neutrons] Abundance: synthetic Half life: 39.8 days [ beta- ] Decay Energy: 0.288MeV Decays to Fm. 255 Half life: 39.8 days [ Alpha Decay ] Decay Energy: 6.436MeV Decays to 251 Bk. Half life: 39.8 days Decay Energy: ?MeV Decays to ?. Isotopes of Fermium Notes Fermium has no stable isotopes. Notable Isotopes Fm [152 neutrons] 252 Abundance: synthetic Half life: 25.39 hours Decay Energy: ?MeV Decays to ?. Half life: 25.39 hours [ Alpha Decay ] Decay Energy: 7.153MeV Decays to 248 Cf. 253 Fm [153 neutrons] Abundance: synthetic Half life: 3 days [ Electron Capture ] Decay Energy: 0.333MeV Decays to 253 Es. Half life: 3 days [ Alpha Decay ] Decay Energy: 7.197MeV Decays to 249 Cf. Fm [155 neutrons] 255 Abundance: synthetic Half life: 20.07 hours Decay Energy: ?MeV Decays to ?. Half life: 20.07 hours [ Alpha Decay ] Decay Energy: 7.241MeV Decays to 251 Cf. Fm [157 neutrons] 257 Abundance: synthetic Half life: 100.5 days [ Alpha Decay ] Decay Energy: 6.864MeV Decays to 253 Cf. Half life: 100.5 days Decay Energy: ?MeV Decays to ?. Isotopes of Mendelevium Notes Mendelevium has no stable isotopes. Notable Isotopes 257 Md [156 neutrons] Abundance: Synthetic Half life: 5.52 hours [ Electron Capture ] Decay Energy: 0.406MeV Decays to Fm. 257 Half life: 5.52 hours [ Alpha Decay ] Decay Energy: 7.558MeV Decays to 253 Es. Half life: 5.52 hours Decay Energy: ?MeV Decays to ?. 258 Md [157 neutrons] Abundance: Synthetic Half life: 51.5 days [ Electron Capture ] Decay Energy: 1.230MeV Decays to 259 Fm. 258 Md [158 neutrons] Abundance: Synthetic Half life: 31.8 days Decay Energy: ?MeV Decays to ?. Half life: 31.8 days [ Alpha Decay ] Decay Energy: 7.000MeV Decays to 256 Es. Half life: 31.8 days [ Electron Capture ] Decay Energy: ?MeV Decays to Fm. 260 Half life: 31.8 days [ beta- ] Decay Energy: 1.000MeV Decays to 260 No. Isotopes of Nobelium Notes Nobelium has no stable isotopes. Notable Isotopes 253 No [151 neutrons] Abundance: synthetic Half life: 1.7 minutes [ Alpha Decay ] Decay Energy: 8.440MeV Decays to Fm. 249 Half life: 1.7 minutes [ Electron Capture ] Decay Energy: 3.200MeV Decays to 255 253 Md. No [153 neutrons] Abundance: synthetic Half life: 3.1 minutes [ Alpha Decay ] Decay Energy: 8.445MeV Decays to Fm. 251 Half life: 3.1 minutes [ Electron Capture ] Decay Energy: 2.012MeV Decays to 259 255 Md. No [157 neutrons] Abundance: synthetic Half life: 58 minutes [ Alpha Decay ] Decay Energy: 7.910MeV Decays to Fm. 255 Half life: 58 minutes [ Electron Capture ] Decay Energy: 0.500MeV Decays to 259 Md. Half life: 58 minutes Decay Energy: ?MeV Decays to ?. Isotopes of Lawrencium Notes Its most stable isotope is Lr, with a half-life of approximately 4 hours. 262