Chapter 28
advertisement

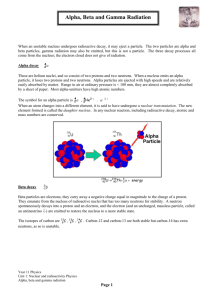
Chapter 28 Nuclear Chemistry Special Note We are not going to cover Section 4 in Chapter 28. We are only going to cover a bit of Section 3, really just a definition of nuclear fusion and fission. You can ignore the “Nuclear Waste” section of Chapter 3. Those parts do make interesting reading, but they will NOT be on my test. Objective A http://pain.health-info.org/pictures/Xray%27s/Xray.head.side.jpg What is radioactivity? What is radiation? What is radioactive decay? X-ray is a type of radiation (that’s what “ray” means). “X” just means unknown, because when it was discovered it was something new, and so it was called an X-ray. The name stuck. Objective A http://www.liveindia.com/news/09oct08e.jpg These things all sound really bad and really dangerous. But they are not. These things are all associated with nuclear reactions. Nuclear reactor in India. The last nuclear reactor built in the US was constructed in 1977. Objective A http://goatmilk.files.wordpress.com/2008/05/mushroom-cloud1.jpg Nuclear reactions are just reactions that occur in the nucleus. Chemical reactions occur by electrons interacting with each other. Nuclear reactions can release a lot of energy, as seen in the photograph. However, to do that, you need a “critical mass” of radioactive material. That’s usually quite a lot. mc2. E= c is the speed of light and c2 is a HUGE number. So even a little bit of mass can convert to a LOT of energy. In a nuclear reaction, an element CAN change into another kind of element. This doesn’t violate Dalton’s theory, because it’s not a chemical reaction. Objective A http://z.about.com/d/chemistry/1/0/0/R/uranium.jpg http://www.vanderkrogt.net/elements/images/lead_dutch_boy_paperweight.jpg 238U decays in a series of 14 steps to 206Pb. Lead is stable. Nuclear reactions occur for one reason and one reason only: the nucleus is unstable. They become stable by giving off radiation, and ultimately by changing into a more stable nucleus. When the nucleus becomes stable, the nuclear reaction is over. Objective A http://exoplanet.as.arizona.edu/~lclose/teaching/a202/radioactive-atom.gif Radioactive decay is the process by which unstable nuclei (plural of nucleus) become stable. α = alpha β = beta γ = gamma They become stable by giving off radiation (particles or energy or both). Radiation or nuclear radioactivity comes in 3 types: alpha, beta and gamma. Objective B http://www.zamandayolculuk.com/cetinbal/AE/alpha.jpg Alpha particles are the same as a helium nucleus. It has 2 protons and 2 neutrons. It has no electrons, and so it has a +2 charge. Since an α particle has 2 protons, Z = 2. Since it also has 2 neutrons, the mass number is 4. Objective B http://www.impcas.ac.cn/usr/wjx/zhonglz/jiangzuo/prc/alpha_decay.gif Z = 95 Z = 93 So when a nucleus loses an alpha particle (called alpha radiation), it loses 2 protons and 2 neutrons. It changes into a DIFFERENT element. It’s atomic number decreases by 2. It’s mass decreases by 4. Objective B Beta particles are basically just an electron. The mass number of a beta particle is 0. For the atomic number, we say that it is “-1.” We say it’s -1, because losing a beta particle causes the nucleus to GAIN a proton. We’ll see how in a little bit. Yes, it’s in German. Not all the best stuff is in English. Notice that an electron is “produced” when a neutron splits into a proton and an electron. Being negative, the electron is immediately spit out of the positive nucleus. “Strahlung” means radiation. Don’t worry about the antineutrino. We won’t worry about that for now. Objective B http://www.atomicarchive.com/Physics/Images/beta.jpg So when a nucleus loses a beta particle (called β radiation), it GAINS one proton and the mass remains the same. It changes into a DIFFERENT element (in this case from H to He). Objective B http://www.lbl.gov/abc/wallchart/chapters/03/3.html The third type of radiation is gamma radiation. Gamma is NOT a particle, like alpha or beta. Gamma rays (or γ radiation) is pure energy. It has 0 mass and the atomic number is 0 as well. Objective B http://www.tartareandesire.com/bands/images/gamma_ray.jpg A German band… In 1988 Kai Hansen left his band Helloween since he was tired of the bad atmosphere in and around the band. Together with Dirk Schlächter and Ralf Scheepers he formed a new band called Gamma Ray. Who will have them on iPod by the end of this unit?? Gamma radiation is often released along with alpha or beta radiation. The nucleus loses energy (it’s this energy that can be harnessed to do productive work…like nuclear power plants…or destructive things, like an atomic bomb). Objective B http://www.crystalxp.net/galerie/img/img-wallpapers-gamma-radiation-atomnet-15582.jpg It came up when I searched on γ radiation. I just thought it looked cool. When the nucleus releases gamma radiation, the mass doesn’t change and the element’s identity doesn’t change either. Only alpha and beta radiation cause the element to turn into a different element. Objective B http://www.users.zetnet.co.uk/mongsoft/images/radiation%20suit.jpg We are constantly being bombarded with alpha, beta and gamma radiation. Radiation suit for protection; luckily, we don’t have to wear these! You don’t know it and you can’t stop it. Luckily, the levels are so low naturally that it doesn’t cause us any kind of harm. Objective B http://www.sciencegeek.net/Chemistry/Unit1/300px-Alfa_beta_gamma_radiation.svg.png Alpha particles have the LEAST amount of energy. Paper can stop alpha particles. Beta particles have more energy than alpha, but less than gamma. Aluminum foil or a thin piece of wood can stop beta particles. Gamma particles have the most energy by far. Several meters of concrete will stop them as will several centimeters of lead. They easily pass through the human body, of course. Objective B http://www.lbl.gov/abc/Basic.html#Nuclearstructure You have to be able to write nuclear reactions. Luckily, they are very easy, if you can do some simple arithmetic. Alpha radiation Mass: 263 = 259 + 4 263Sg 259Rf + 4α Z: 106 = 104 + 2 Beta radiation Mass: 14 = 14 + 0 14C 14N + 0β Z: 6 = 7 + (-1) Enrico Fermi, an Italian Nobel Prize winner, who worked on nuclear reactors and quantum mechanics. Objective C http://www.wbabin.net/science/imani3.pdf https://salksperiodictable.wikispaces.com/file/view/fermium.001.png There are 109 elements, but over 1,500 different possible isotopes for those elements. Of those, only about 264 are stable. The rest decay, by some form radioactive decay, to BECOME stable. How fast they decay is dependent on the specific isotope. Some decay rapidly…in seconds. Fermium-258 has a half life of 0.00038 seconds (I found this in an Iranian chemical journal…science really is a universal language.) Other isotopes take billions of billions of years to decay. Objective C http://en.wikipedia.org/wiki/Bismuth The longest known half life is for 209Bi which has a half life of 1.9 x 1019 years or 19,000,000,000,000,000,000 years. (That’s 426,000,000,000 times LONGER than the “accepted” age of the Universe.) As a matter of fact, I used to tell my students that 209Bi was the largest isotope (in terms of mass) which was stable. Bismuth crystals. Pretty cool, huh? Bismuth-209 isn’t stable at all. It just decays so slowly that it appears to be stable to us. Schwartz’s Hypothesis on Nuclear Decay http://en.wikipedia.org/wiki/Iron It got me to thinking… What if EVERYTHING decays? But what if some things decay sooooo slowly as to be all but impossible to measure it. Iron pillar in India which has withstood corrosion for over 1,600 years. It’s my hypothesis. I don’t know how to design an experiment to prove it yet though. Fe is thought to be “the most stable” element. It is the heaviest element formed by fusion in stars. Every element above Fe is slowly decaying until it becomes Fe? Objective C http://www.physics.isu.edu/radinf/natural.htm Isotope Half Life Information Carbon-14 5,730 years Used in dating of ancient artifacts Tritium (H-3) 12.3 years Produced from weapons testing Iodine-131 8.04 days Used to treat thyroid disease Technetium-99 2.11 x 105 years Beta decay product of Mo-99. Used for medical diagnoses. Used as a γ-free source of β particles. Many other things have half lives of minutes or days or years or decades or centuries, or even millions of years. How do you know IF an isotope is stable. Let’s talk about the Band of Stability. The stable isotopes can be calculated using a simple formula. The Band of Stability #N/#P = 1.5 Find the value of # Neutrons / # Protons If that number is greater than or equal to 1 AND less than or equal to 1.5, the isotope is stable. So, anything less than 1 is unstable. Anything greater than 1.5 is unstable. The Band of Stability This formula really only works for elements with an atomic number > 20. For example, 14C is radioactive, but the ratio 8 N / 6 P = 1.33. Elements with Z < 20 generally decay by β decay. Elements with Z > 83 are always radioactive. In other words, for Po (Polonium, named by Marie Curie for her native Poland) and higher there are NO naturally occurring stable isotopes. Elements with a HIGH atomic number that are unstable usually have too many neutrons. They decay by α decay, primarily until they reach 206Pb, which is a stable isotope of lead. Transmutation http://s185.photobucket.com/albums/x266/glenlantz/?action=view&current=chfa_04_img0924.jpg&newest=1 Elements become other elements in something called a transmutation rxn. Radioactive decay is a transmutation process. Nuclear experiments have successfully transmuted lead into gold, but the expense far exceeds any gain. So modern chemistry has succeeded where the alchemists could not. In fact, it’s far easier to turn gold into lead. Objective C http://www.jeopardy.com/multimedia_downloads.php Transuranium elements are the elements that have an atomic number greater than U. Know why Plutonium was abbreviated Pu and not Pl? Basically, because top scientists really are geeks. At least, so said Alex Trebek on Jeopardy! Remember when you were a kid, and you said “P-u.” That’s why. It probably made him laugh every time he thought of it. All of the transuranium elements are man-made. Most of them only exist for a second or less and then they decay into something else. Many of them were made by Dr. Seaborg and his team of researchers. 93Np for example decays by beta decay into 94Pu, which then decays by α decay into 92U. Uranium then decays in a series of 14 steps to 206Pb. Beta Decay: where does the electron come from? In a reaction form, beta decay can be expressed as 0n -1β + 1p The neutron has a mass of 1 and an “atomic number” of 0. When the beta particle forms it is “spit out” of the nucleus. The proton that was formed remains in the nucleus. Since Z goes up by 1, it is now a new element. Objective D There is also a process where a nucleus can emit something called a “positron.” When this happens, the mass remains the same but the atomic number goes down by 1. A positron is similar in size to an electron, and similar in mass, but it has a positive charge, and an atomic number of 1. Objective D In a reaction form, positron emission can be expressed as 1p +1β + 0n Again, the positron is ejected, and the neutron remains in the nucleus. Since the atom lost a proton, Z goes down by 1 and a new element is formed. Objective D http://defaultprime.com/wp-content/uploads/2009/03/halflife2.jpg picture cropped Half life (t½) is the time required for one half of the nuclei in a radioactive sample to decay. As we have seen, half lives can range from fractions of a section to billions of years. Big Hairy, Easier than it Looks Formula Alert Half-Life Calculations A = A0(½)t/T Where A = amount at time = t. A0 = original amount at time = 0. t = elapsed time T = half life (same units as “t”) Sounds simple enough… El Samplo Problemo hey, I took French in high school Co-60 decreases from 0.800 g to 0.200 g in a period of 10.5 years. From this information, what is the half life. A…let’s not use the formula B…let’s do use the formula After one half life, half should have decayed. 0.800 g 0.400 g El Samplo Problemo hey, I took French in high school Easy! Co-60 decreases from 0.800 g to 0.200 g in a period of 10.5 years. From this information, what is the half life. A…let’s not use the formula After one half life, half should have decayed. 0.800 g 0.400 g After two half lives, we find ourselves at the amount listed in the problem. 0.800 g 0.400 g (1st half life) 0.400 g 0.200 g (2nd half life) So, 10.5 years = 2 half lives. Therefore 5.25 years = 1 half life. El Samplo Problemo hey, I took French in high school Easy, too! Co-60 decreases from 0.800 g to 0.200 g in a period of 10.5 years. From this information, what is the half life. B…let’s do use the formula A = A0(½)t/T A = 0.200 g; A0 = 0.800 g; T= ? and t = 10.5 years El Samplo Problemo hey, I took French in high school Easy, too! But you do need to know logs. Sorry! That Algebra 2 Ok, so I lied. Some of you won’t think it’s easy! Co-60 decreases from 0.800 g to 0.200 g in a period of 10.5 years. From this information, what is the half life. B…let’s do use the formula 0.200g = 0.800g x (½)10.5 years/T 0.25 = (½)10.5 years/T Ok, I divided both sides by 0.800 to get this El Samplo Problemo hey, I took French in high school Easy, too! 0.25 = (½)10.5 years/T Log (ax) = x times log(a) log(0.25) = log[(½)10.5 years/T] So from where we left off, take the log of both sides. Use the log rule here -0.602 = 10.5years/T x log(½) -0.602 = 10.5years/T x (-0.301) 2 = 10.5years/T 2 x T = 10.5 years or T = 5.25 years Second Objective D (yes, it should be Objective E, but it’s not, but I just want you to know these two definitions) http://hyperphysics.phy-astr.gsu.edu/Hbase/nucene/u235chn.html Nuclear fission is the splitting of a large nucleus into smaller fragments. They break apart to form smaller elements. U-235 combined with a neutron to split into Ba-144 and Kr-89 + 3 neutrons. Those neutrons can then react with other U-235 atoms, causing a “chain reaction.” Note the 235 + 1 = 89 + 144 + 3 Second Objective D (yes, it should be Objective E, but it’s not, but I just want you to know these two definitions) http://www.asi.org/adb/02/09/he3-intro.html Nuclear fusion is when smaller nuclei combine to produce a larger nucleus. They “fuse” together, like if you take 2 pieces of clay and “mush” them together. H-2 + He-3 p + He-4 + a LOT of energy… 25 tons of He-3 could replace 227.3 MILLION tons of fossil fuels with no pollution and no harmful radiation! There is some interesting stuff in the study guide on nuclear fusion and He-3 on the moon. And for more information, check out the link above (the Artemis project). The End Make sure you read the entire webpage at the Chapter 28 stuff link.