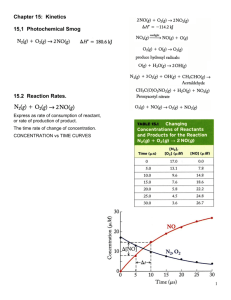
Kinetics: Ch 28 and 29
advertisement

Chemical Kinetics CK-1 Chemical kinetics is the study of time dependence of the change in the concentration of reactants and products. “The field of chemical kinetics has not yet matured to a point where a set of unifying principles has been identified…There are many different theoretical models for describing how chemical reactions occur.” (M&S, 1137) Generally, we want to understand the rate of reaction: d [reactant ] Rate Reactant Product dt Recall from Slide CEq-2… CK-2 vA A( g ) vB B( g ) vY Y ( g ) vZ Z ( g ) Reactants Products nA (t ) nA (0) vA (t ) nY (t ) nY (0) vY (t ) nB (t ) nB (0) vB (t ) nZ (t ) nZ (0) vZ (t ) For each constituent… 1 dn A d [ A] v A d (t ) V dt dt V dt Rate of reaction, v(t) CK-3 v(t), the rate of reaction, is defined as the rate of change in ξ(t) with time per unit volume 1 d (t ) 1 d [ A] 1 d [ B] 1 d [Y ] 1 d [ Z ] v(t ) V dt v A dt vB dt vY dt vZ dt Note all quantities are positive. Examples: What are units of v(t)? 2NO(g) + O2 (g) 2NO2 (g) 1 d (t ) v(t ) V dt H2(g) + I2 (g) 2HI (g) 1 d (t ) v(t ) V dt The integrated rate law CK-4 The rate can be expressed as a function of the reactant concentrations. Most common function is of form: 1 d v(t ) k[ A]m [ B]n V dt Rate laws must be determined experimentally and, generally, cannot be deduced from the balanced reaction!! General vA A( g ) vB B( g ) vY Y ( g ) vZ Z ( g ) v(t ) k[ A] [ B] m n Example 2NO(g) + O2 (g) 2NO2 (g) v(t ) k[ NO]2 [O2 ] Determined experimentally! The Order: mth order in A nth order in B (m+n)th order overall Finding rate laws experimentally CK-5 There are two common methods for determining rate laws: Method of isolation Set up reaction so one reactant is in excess. Any change in rate will be due to changes in other reactant. Repeat for other reactant. v k [ B] n where k k[ A] m Method of initial rates Measure concentration change as a function of time, ~v(t), for a series of experimental conditions. (Conditions must include sets where the reactant A has the same initial concentration but B changes and vice versa). Units of k, rate constant CK-6 1 d m n v(t ) k[ A] [ B] V dt (concentration) n concentration time Rate Law (concentration)m Order 0 Units of k v=k 1 v =k[A] 2 v = k[A]2 v = [A][B] 1 in [A], [B] 2 overall v = k[A]1/2 1/2 v(t ) (concentrat ion )1mn k m n [ A] [ B] time Rate laws can be complicated H2(g) + I2 (g) 2HI (g) CK-7 v(t ) k[H2 ][I2 ] 2k [H 2 ][ Br2 ]1/ 2 H2(g) + Br2 (g) 2HBr (g) v(t ) 1 k [HBr ][ Br2 ]1 These rate laws suggest that these two reactions occur via different mechanisms (sets of individual steps). The first may be a elementary reaction (one step) whereas the latter is certainly a multistep process. We will soon explore how to obtain complicated rate laws from suggested mechanisms. Elementary Rxns vs. Complex Reactions CK-8 Complex Reactions Elementary Reactions Reactants Intermediates Products Reactants Products Mechanism of a complex reaction is a sequence of elementary reactions. Molecularity of Elementary Reactions: Unimolecular A products Bimolecular A B products Termolecular A B C products First order reactions The reaction: A B products CK-9 d [ A] has rate law: v(t ) k[ A] dt Let’s integrate… t 1 [ A]0 [ A] d[ A] 0 kdt d [ A] kdt [ A] [ A ]t Solution: [ A]t ln kt [ A]0 ln[ A]t ln[ A]0 kt [ A]t [ A]0 e kt First order reactions decay exponentially. Ozone decays via first order kinetics CK-10 O3 ( g ) O2 ( g ) O( g ) k = 1.078 × 10-5 s-1 at 300 K [O3 ]t [O3 ]0 e kt [O 3 ]t ln kt [O 3 ]0 What is slope? What happens as k increases? [ A]t [ A]0 e kt k = 0.0125 s-1 k = 0.0250 s-1 k = 0.0500 s-1 k = 0.1000 s-1 [ A]t ln kt [ A]0 CK-11 Half-life of a first order reaction CK-12 The half-life, t1/2, is the time it takes to fall to ½ of the starting concentration: At t t1/ 2 , [ A]t1 / 2 1 [ A]0 2 [ A]1/ 2 1 e kt1 / 2 [ A]0 2 Figure 28.3 Other order reactions… Second order reaction: Second order rate: 2 A products 1 d [ A] v(t ) k[ A]2 2 dt Integrated rate law: 1 1 2kt [ A]t [ A]0 CK-13 A B products v(t ) [ B]0 [ A]t 1 ln [ A]0 [ B]0 [ A]0 [ B]t Zero order reaction: A products Zero order rate: v(t ) Integrated rate law: d [ A] k[ A][ B] dt d [ A] k dt [ A]t [ A]0 kt kt Pseudo-first order reactions CK-14 You can “overload” the other reactants to determine the order with respect to one individual reactant (method of isolation). For A B products , what happens if [B] >> [A]? d [ A] v(t ) k[ B ][ A] dt Similar to SN2 Lab Reversible reactions (small DrG) A k1 k-1 B CK-15 Assume first order, elementary rxn in both directions d [ A] k1[ A] k 1[ B] dt Rate: Conservation of Mass: [ A]0 [ B]0 [ A] [ B] [ B] [ A]0 [ B]0 [ A] d [ A] k1[ A] k 1 [ A]0 [ B]0 [ A] dt Integrate: k1[ A]t k1 [ A]0 [ B]0 [ A]t (k1 k1 )t ln k1[ A]0 k1[ B]0 At equilibrium A k1 k-1 B CK-16 d [ A] k1[ A] k 1[ B] dt d [ A ] At equilibrium… 0 dt k1[ A]eq k 1[ B]eq The forward rate equals the reverse at equilibrium. What is the equilibrium constant for this reaction? In terms of rate constants? k1 K eq k 1 K eq [ B]eq [ A]eq Temperature Dependence of k CK-17 The rate constant can vary in different ways with T. Svante Arrhenius Winner of the 3rd Nobel Prize in Chemistry Differential form of the Arrhenius Equation: Ea d ln k 2 dT RT Arrhenius Parameters CK-18 Integrated forms of Arrhenius equation: Ea ln k ln A RT k Ae Ea / RT Activated (or transition) state Ea is the activation energy. This is the energy required to get over a barrier (at the activated or transition state) between the reactants and products. Ea has units of energy and is T independent. A is the pre-exponential or Arrhenius factor and is T dependent. A is a measure of rate at which collisions occur (and takes lots of things into acct such as orientation, molecular size, number of molecules per volume, molecular velocity, etc). 2HI(g)→I2(g) + H2(g) Transition-State Theory CK-19 AB‡ is the transition state (or activated complex.) Transition state theory assumes that the transition state and reactants are in equilibrium with each other, and uses concepts from chemical equilibrium and statistical mechanics to find kinetic info such as rate constants! Eyring Equation (key to transition-state theory) From CEq: K e ‡ DG ‡ / RT Change in Gibbs energy from reactants to TS So… k BT k e h k BT ‡ k KC h ‡ DG / RT DG DH TDS k BT DS ‡/ R DH‡ / RT k e e Enthalpy of activation h Entropy of activation Relating Ea to thermodynamics! CK-20 Necessary Pieces… Arrhenius Equation: ln k ln A Ea d ln k Differentiate wrt T: dT RT 2 Ea RT or d ln k Ea RT dT 2 ‡ From Eyring Equation: d ln k 1 d ln K C dT T dT van’t Hoff Equation (for Kc): Putting it all together… d ln K C DU dT RT 2 ‡ 1 d ln K C Ea RT dT T 2 or Ea RT DU ‡ What about A, the pre-exponential? Ea RT DU ‡ so CK-21 DU ‡ DH‡ RTD‡ng and ‡ ‡ Ea RT DH RT D n Unimolecular Gas Phase Reaction AA‡Products so k BT DS k e h ‡ Ea RT DH ‡ ‡ / R DH / RT e k BT DS ‡ / R Ea / RT k e e e h Bimolecular Gas Phase Reaction A+BAB‡Products so Ea DH 2 RT k BT DS ‡/ R E A / RT k e e e h 2 Same for reactions in solution ‡ What is A? Transition State Theory and NMR Lab CK-22 In the NMR/N,N-DMA Paper, Gasparro et al. found an activation energy of 70.3 kJ/mol and a pre-factor of 1.87 × 1010 s-1. Using these values, and a temperature of 298 K, find… DH ‡ DS ‡ DG ‡ Why is TST important? 1. Provides details of a reaction on the molecular scale. 2. Connects quantum mechanics and kinetics. 3. Currently used for many computational studies on reaction rates. EX-CK3 CK-23 Chapter 29: Reaction Mechanisms Always remember…. CK-24 • One can never prove a reaction mechanism, although evidence may disprove a mechanism. • Verifying proposed mechanisms requires extensive experimental verification of each proposed step! Let’s examine a reaction … CK-25 A P k o bs Reaction could progress in multiple ways… How can we distinguish? Case 1: One elementary step A P k1 Case 2: Two step reaction A I k1 I P k2 EX-CK4 Now let’s focus on the intermediate… CK-26 A I P k1 k2 How do k1 and k2 relate in case a? in case b? (a) I forms quickly but decays slowly… k1 is fast relative to k2. (b) I builds up to a constant, nearly negligible, concentration until near end of reaction. … k1 is slow relative to k2. EX-CK5 d[I ] 0 dt Steady state approximation... Valid only if k2 > k1. Rate Laws do not yield unique mechanisms CK-27 An empirically determined rate law does not imply a unique reaction mechanism! Consider reaction: obs 2NO( g ) O2 ( g ) k 2NO2 ( g ) Experimentally, it was determined that the rate is given by: v(t ) kobs[ NO] [O 2 ] 2 Researchers proposed two possible mechanisms. They need to determine if one of them is correct. So how would researchers distinguish between the mechanisms? EX-CK6 Remember the Chain Rxn from CK-6? CK-28 1/ 2 2 k [ H ][ Br ] 2 2 H2(g) + Br2 (g) 2HBr (g) v(t ) 1 1 k [HBr ][ Br2 ] Proposed Mechanism Initiation: Propagation: k1 Br2 ( g ) M ( g ) 2Br ( g ) M ( g ) k2 Br ( g ) H 2 ( g ) HBr ( g ) H ( g ) k3 H ( g ) Br2 ( g ) HBr ( g ) Br ( g ) Inhibition: k 2 HBr ( g ) H ( g ) Br ( g ) H 2 ( g ) Termination: k 1 2Br ( g ) M ( g ) Br2 ( g ) M ( g ) EX-CK7 What about the solution kinetics lab?! CK-29 • Now that we’ve explored reaction mechanisms and rate laws, let’s try to derive the rate laws from the solution kinetics lab… EX-CK8 Catalysis CK-30 Catalyst: A substance that participates in the chemical reaction but is not consumed. Provides a new mechanism for reaction and can cause reaction to occur faster. In an experiment involving a catalyst, there are two competing reactions: A products EX-CK9 A catalyst products catalyst If both reactions are elementary, overall rate is given by: d [ A] k[ A] kcat [ A][catalyst ] dt