CHAPTER 4: IMPERFECTIONS IN SOLIDS
advertisement

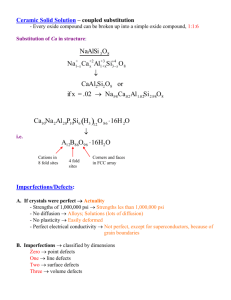
CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS... • What types of defects arise in solids? • Can the number and type of defects be varied and controlled? • How do defects affect material properties? • Are defects undesirable? • How do point defects in ceramics differ from those in metals? • In ceramics, how are impurities accommodated in the lattice and how do they affect properties? 1 Imperfections in Solids • Introduction and Motivation – Why study this? • What I have shown you so far are “idealized” structures • You would get the impression that solids (particularly crystalline solids) have “perfect” structures – This is clearly not the case • All solids have imperfections, and these have profound influences on mechanical (e.g. alloys of Ag/Cu v pure silver), electrical (e.g. doping of silicon with boron) properties • “Crystalline defect” – a lattice irregularity having one or more of its dimensions on the order of an atomic distance • We will also organize this discussion around whether the defect involves a single atom (0 D), rows of atoms (1D), or interfacial (2D) defects TYPES OF IMPERFECTIONS • Vacancy atoms • Interstitial atoms • Substitutional atoms Point defects • Dislocations Line defects • Grain Boundaries Area defects 2 Imperfections in Solids • Point defects in metals – Start simple: what happens when you have a crystal structure and one of the atoms is missing vacancy – Every crystal has vacancies – Why? From a thermodynamic viewpoint, crystalline solids have very low entropy (why?) – The introduction of vacancies increases the entropy of the crystal – Can estimate the number of vacancies in a crystal using a Boltzmann type description Qv N v N exp k BT Qv is the energy required for forming a vacancy For a “typical” metal Nv/N slightly below the melting temperature is ~ 10-4 POINT DEFECTS • Vacancies: -vacant atomic sites in a structure. distortion of planes Vacancy • Self-Interstitials: -"extra" atoms positioned between atomic sites. distortion of planes selfinterstitial 3 EQUIL. CONCENTRATION: POINT DEFECTS • Equilibrium concentration varies with temperature! No. of defects Activation energy Q ND exp D kT N No. of potential Temperature defect sites. Boltzmann's constant (1.38 x 10 -23 J/atom K) (8.62 x 10 -5 eV/atom K) Each lattice site is a potential vacancy site 4 MEASURING ACTIVATION ENERGY • We can get Q from an experiment. • Measure this... • Replot it... ND ln N 1 slope -QD/k 1/T 5 ESTIMATING VACANCY CONC. • Find the equil. # of vacancies in 1m3 of Cu at 1000C. • Given: 0.9eV/atom Q ND exp D = 2.7 · 10-4 kT N For 1m 3, N = • Answer: 1273K 8.62 x 10-5 eV/atom-K NA x 1m3 = 8.0 x 1028 sites x ACu 6 OBSERVING EQUIL. VACANCY CONC. • Low energy electron microscope view of a (110) surface of NiAl. • Increasing T causes surface island of atoms to grow. • Why? The equil. vacancy conc. increases via atom motion from the crystal to the surface, where they join the island. Movie m3 Reprinted with permission from Nature (K.F. McCarty, J.A. Nobel, and N.C. Bartelt, "Vacancies in Solids and the Stability of Surface Morphology", Nature, Vol. 412, pp. 622-625 (2001). Image is 5.75 mm by 5.75 mm.) Copyright (2001) Macmillan Publishers, Ltd. 7 Imperfections in Solids • Point defects in ceramics – Point defects such as vacancies are also present in ceramics – Here it is a bit more involved, since ceramics have both cations and anions – You might imagine that since the anions are (much?) larger than the cations there are very few anion interstitial defects – “Defect structure” is often used to denote defects in ceramics – again need to deal with charge neutrality Imperfections in Solids • Point defects in metals – A void left by an atom in the crystal structure is called a vacancy – An additional atom is called a self-interstitial • This is an atom that is located in one of the interstitial voids in the crystal structure (remember those) that are usually not occupied – These occur much less frequently than defects (why?) Imperfections in Solids • Point defects in ceramics – Because of charge neutrality, defects in ceramics must occur in pairs – Frenkel defect -- cation-vacancy and cation-interstitial pair (cation moves from regular site to interstitial site) – Schottky defect – cation-vacancy and anion-vacancy pair (move one cation and one anion from the lattice to the external surface) DEFECTS IN CERAMIC STRUCTURES • Frenkel Defect --a cation is out of place. • Shottky Defect --a paired set of cation and anion vacancies. Adapted from Fig. 13.20, Callister 5e. (Fig. 13.20 is from W.G. Moffatt, G.W. Pearsall, and J. Wulff, The Structure and Properties of Materials, Vol. 1, Structure, John Wiley and Sons, Inc., p. 78.) See Fig. 12.21, Callister 6e. • Equilibrium concentration of defects ~ e QD / kT 8 Imperfections in Solids • Stoichiometry – Stoichiometric materials are those where the exact cation:anion ratio is predicted from the chemical formula (i.e. NaCl); deviation from this leads to nonstoichiometric materials – How would you get this? • Ions with multiple oxidation states (e.g. Fe+2, Fe+3) • Impurities These types of issues can lead to defects Fe1-xO x<1 Non-stoichiometry Deficiency of Fe Imperfections in Solids • Impurities in solids (metals) – It is very hard to make ultra-high purity metals – Often turns out you don’t want to! – Mixtures of metals (or alloys) often have better properties than the pure compounds – e.g. alloying silver with copper – Some definitions: • Solid solution – single “solid phase” containing two (or more) metals – Formed upon addition of impurity • For binary metal solid solutions – solvent/solute nomenclature used POINT DEFECTS IN ALLOYS Two outcomes if impurity (B) added to host (A): • Solid solution of B in A (i.e., random dist. of point defects) OR Substitutional alloy (e.g., Cu in Ni) Interstitial alloy (e.g., C in Fe) • Solid solution of B in A plus particles of a new phase (usually for a larger amount of B) Second phase particle --different composition --often different structure. 9 Imperfections in Solids • Solid solutions – In the case of a solid solution, upon addition of the impurity to the host material, the crystal structure is maintained – Analogy to liquids • Add two miscible liquids together (water & methanol) – The composition is homogeneous throughout – Same idea with solid solutions – impurity atoms are randomly and uniformly dispersed within the solid ALLOYING A SURFACE • Low energy electron microscope view of a (111) surface of Cu. • Sn islands move along the surface and "alloy" the Cu with Sn atoms, to make "bronze". • The islands continually move into "unalloyed" regions and leave tiny bronze particles in their wake. • Eventually, the islands disappear. Movie m4 Reprinted with permission from: A.K. Schmid, N.C. Bartelt, and R.Q. Hwang, "Alloying at Surfaces by the Migration of Reactive TwoDimensional Islands", Science, Vol. 290, No. 5496, pp. 1561-64 (2000). Field of view is 1.5 mm and the temperature is 290K. 10 Imperfections in Solids • Substitutional solid solutions – Regarding substitutional defects, there are several features that determine how well the impurity dissolves in the host material 1. Atomic size factor – close in size, the better; if the difference is more than about +/-15%, form new solid phases Crystal structure – crystal structures for both species should be the same (i.e. hard to dissolve an FCC metal in a BCC metal) Electronegativity – closer the better; if the difference between the two is large, they will likely form an intermetallic compound instead of a substitutional solid solution Valence – Metal will have a tendency to dissolve a metal of higher valency versus lower valency 2. 3. 4. – Example: Cu and Ni are “model” substitutional solid solution system • Size similar, both FCC, similar electronegativities, Ni+2, Cu+1 Imperfections in Solids • Interstitial solid solutions – Impurity atoms fill voids or interstices among the host lattice – Given the high packing factors in metal structure (bcc, fcc, hcp), the size of these positions is small – So, dinterstitial atom < dhost atom – Also very few of these defects (why?) – Example – Carbon forms an interstitial solid with iron (what is this?) – however can only add 2% carbon Imperfections in Solids • Impurities in Ceramics – Can also have impurities in ceramics (again, issue of anions v cations, electroneutrality) IMPURITIES • Impurities must also satisfy charge balance • Ex: NaCl • Substitutional cation impurity • Substitutional anion impurity O2- initial geometry ClClO2- impurity anion vacancy resulting geometry 11 Imperfections in Solids • Sections 5.5, 5.6 – Read – 5.5 – can have point defects in polymers – 5.6 – composition units conversion, you should know all of this already from 204(?) COMPOSITION Definition: Amount of impurity (B) and host (A) in the system. Two descriptions: • Weight % • Atom % • Conversion between wt % and at% in an A-B alloy: CB = C'BAB C'AAA + C'BAB x 100 • Basis for conversion: 12 Imperfections in Solids • Dislocations/linear defects – A dislocation is a linear (one-dimensional) defect around which atoms are misaligned – Simplest type of dislocation – the presence of an extra portion of a plane of atoms, which terminates inside the crystal • This is called an edge dislocation This is an extra plane of atoms Note lattice distortion around plane Atoms above dislocation line are squeezed together, those below are pushed apart Magnitude and direction of lattice distortion is expressed in terms of the Burgers vector Imperfections in Solids • Edge dislocation – Few other points – Where the plane ends is often called the “edge dislocation line” denoted by the ┴ symbol – The symbol points up if the extra plane is in the “top” of the crystal; points down if it is the “bottom” of the crystal Also note the Burgers vector b This is perpendicular to the edge dislocation LINE DEFECTS Dislocations: • are line defects, • cause slip between crystal plane when they move, • produce permanent (plastic) deformation. Schematic of a Zinc (HCP): • before deformation • after tensile elongation slip steps 13 Imperfections in Solids • Screw dislocation – Can think of this as being due to a shear stress applied to the crystal – The upper front region of the crystal is shifted one atomic distance to the right – This also can be thought of as a linear dislocation along the line AB in the bottom Figure Imperfections in Solids • Screw dislocation – Atomic positions above the “slip plane” are open circles, those below are solid circles – Here the Burgers vector b is parallel to the screw dislocation In reality, most defects have components of both types … these are referred to as mixed dislocations INCREMENTAL SLIP • Dislocations slip planes incrementally... • The dislocation line (the moving red dot)... ...separates slipped material on the left from unslipped material on the right. Simulation of dislocation motion from left to right as a crystal is sheared. Click on image to animate (Courtesy P.M. Anderson) 14 BOND BREAKING AND REMAKING • Dislocation motion requires the successive bumping of a half plane of atoms (from left to right here). • Bonds across the slipping planes are broken and remade in succession. Atomic view of edge dislocation motion from left to right as a crystal is sheared. (Courtesy P.M. Anderson) Click on image to animate 15 Imperfections in Solids • Interfacial defects – We have now talked about zero-dimensional (i.e. point) and onedimensional (linear) defects. Can also have two-dimensional defects – These are boundaries that normally separate regions of the material with different crystal structures and/or crystallographic orientation (remember about grains?) – The most common – external surfaces, grain boundaries, stacking faults, twin boundaries, and phase boundaries Imperfections in Solids • Interfacial defects – External surfaces • Why defects? Surface atoms are not bonded to the same number of atoms as the “bulk” atoms • They are therefore in a higher energy state • Nature (thermodynamics) says minimize your free energy (here dominated by surface free energy effects) • In liquids – typically get spherical droplets (why?) – Solids can’t do this • However, it is common to see structural rearrangement at surfaces to minimize energy Imperfections in Solids • Interfacial defects – Grain boundaries • Boundaries between domains (or grains) of a polycrystalline material that possesses different crystallographic orientations • Within the boundary there is mismatch of the atomic arrangements as compared to the crystallographic domains • Orientation mismatch can be minor (small- or low-angle grain boundaries) or major (large- or high-angle grain boundaries) AREA DEFECTS: GRAIN BOUNDARIES Grain boundaries: • • • • are boundaries between crystals. are produced by the solidification process, for example. have a change in crystal orientation across them. impede dislocation motion. Metal Ingot Schematic ~ 8cm grain boundaries Adapted from Fig. 4.7, Callister 6e. heat flow Adapted from Fig. 4.10, Callister 6e. (Fig. 4.10 is from Metals Handbook, Vol. 9, 9th edition, Metallography and Microstructures, Am. Society for Metals, Metals Park, OH, 1985.) 16 Imperfections in Solids • Interfacial defects – Grain boundaries • Tilt boundary – series of edge dislocations leading to a small-angle grain boundary Key point here – the edge dislocations are aligned with respect to one another Imperfections in Solids • Interfacial defects – Grain boundaries • Turns out that atoms along the grain boundary have less regular bonding as compared to that of the “bulk” – This is analogous to surface atoms • • • • As a result they have a higher energy Tend to be more reactive Often impurity atoms will segregate into/along the grain boundaries Grow at elevated temperatures to reduce the total boundary energy Imperfections in Solids • Interfacial defects – Twin boundaries • Special type of grain boundary – across the grain boundary there is a mirror symmetry The region between the boundary is called a twin These are formed due to applied shear forces (mechanical twins) or during high temperature heat treatments (annealing twins) These twins occur on a definite crystallographic plane and in a specific direction Imperfections in Solids • Interfacial defects – Stacking faults • Exactly what it sounds like … the layers in a structure are stacked incorrectly • For example, in the FCC structure an interruption of the ABCABC.. stacking sequence of close-packed planes – Phase boundaries – domains between solids that phase separated (multiphase materials) Imperfections in Solids • Bulk/volume defects – – – – – – These are “3-D” defects Pores – holes in the material Cracks “Foreign inclusions” – a large impurity domain? Usually result from processing/fabrication All effect material properties • Atomic vibrations – read • Microscopic examination – read – Key point – microscopy allows you to generate real space images of solids – Resolution of the image (i.e. size of the objects/features you can see) depends on the wavelength of the radiation used Cu atomic shells for (Cu0.25Ni0.75)343 at various temperatures during the heating process (MD simulations, S-P Huang and PB Balbuena, J. Phys. Chem. B, 2002) 0K 400 K 200 K 700 K 300 K 1300 K Cu0.25Ni0.75 nanocluster (343 atoms) deposited on graphite (not shown) (S-P Huang, D. S. Mainardi and P. B. Balbuena, Surf. Sci., 2003) 700 K 900 K 800 K 1200 K OPTICAL MICROSCOPY (1) • Useful up to 2000X magnification. • Polishing removes surface features (e.g., scratches) • Etching changes reflectance, depending on crystal orientation. close-packed planes Adapted from Fig. 4.11(b) and (c), Callister 6e. (Fig. 4.11(c) is courtesy of J.E. Burke, General Electric Co. micrograph of Brass (Cu and Zn) 0.75mm 17 OPTICAL MICROSCOPY (2) Grain boundaries... • are imperfections, • are more susceptible to etching, • may be revealed as dark lines, • change direction in a polycrystal. Adapted from Fig. 4.12(a) and (b), Callister 6e. (Fig. 4.12(b) is courtesy of L.C. Smith and C. Brady, the National Bureau of Standards, Washington, DC [now the National Institute of Standards and Technology, Gaithersburg, MD].) 18 Electron Microscopy • Instead of light radiation, uses beams of electrons • A high-velocity electron becomes wave-like (QM), and its wavelength is inversely proportional to its velocity. If accelerated (high V), wavelengths could be of ~0.003 nm (3pm). => high magnification and high resolution Electron microscopy • Transmission electron microscope (TEM): an electron beam goes through an specimen (usually prepared as a thin foil) => transmission of a high % of the incident beam. • Transmitted beam is projected for visualization. Magnifications of 1,000,000X or more. (useful for dislocations) Electron microscopy • Scanning electron microscope (SEM): the surface of a specimen is scanned with an electron beam, and the reflected beam of electrons is collected on a cathode ray tube (similar to a TV screen). The surface must be electrically conductive. Magnifications from 10 to 50,000X. SCANNING TUNNELING MICROSCOPY • Atoms can be arranged and imaged! Photos produced from the work of C.P. Lutz, Zeppenfeld, and D.M. Eigler. Reprinted with permission from International Business Machines Corporation, copyright 1995. Carbon monoxide molecules arranged on a platinum (111) surface. Iron atoms arranged on a copper (111) surface. These Kanji characters represent the word “atom”. Uses a tiny probe with a very sharp tip brought in very close proximity of the specimen. The probe 19 movements are monitored, and a 3-D image of the surface is generated. SUMMARY • Point, Line, and Area defects arise in solids. • The number and type of defects can be varied and controlled (e.g., T controls vacancy conc.) • Defects affect material properties (e.g., grain boundaries control crystal slip). • Defects may be desirable or undesirable (e.g., dislocations may be good or bad, depending on whether plastic deformation is desirable or not.) 20 ANNOUNCEMENTS Reading: Chapter 5 HW # 4: Due Monday, February 19th 5.2; 5.7; 5.9; 5.11; 5.27; 5.31; 5.32; 5.34; 5D2 21