Your Report
advertisement

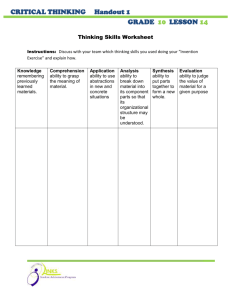
Lab #30 Write-Up (Due 5-16-12) Rubric Your Report Header (Titration of NaOH) Objective Procedure & Materials: Refer to Handout Data: Refer to Tables in Handout Calculations: Show equation, substitution w/units, answer w/units for Discussion: Questions (Neatly answer on back of handout.) Errors/Improvements (Write in report.) Conclusion all titration calculations. Label average value as final answer. Claim – given on handout; Evidence - refer to data and calculations. Science Concept – include questions listed in the handout. Lab Handout & Data Sheet Lab #28 Write-Up (Due 4-25-12) Rubric Your Report Header (Establishing Equilibrium) Objective Procedure & Materials: Refer to Handout Data: Graph attached Discussion: Conclusion Observations # 1-4; Analysis #1-5 (on worksheet) Errors/Improvements Claim – given on handout; Evidence (refer to graph; rate as slope) Science Concept (dynamic equilibrium; rate; concentration; etc.) Lab Worksheet Lab #27 Write-Up (Due 4-16-12) Rubric Your Report Header (Effect Conc. on Rxn Rate) Objective Procedure & Materials: Refer to Handout Data: Tables 1 & 2 (in report) and Graphs 1 & 2 Calculations: Dilution Equation; Instantaneous Rate (Graph 1) Discussion: Conclusion Errors/Improvements and question Claim – given on handout; Evidence (Refer to graph) Science Concept (rate & conc (collision theory); reaction mechanisms; indicators forming a colored complex; activation complex, etc.) Lab Worksheet Lab #26 Write-Up (Due 3-26-12) Rubric Your Report Lab Worksheet (Making a Solution) Show all work for calculations. Lab #25 Write-Up (Due 3-20-12) Rubric Your Report Header (Double Replacement Reactions) Objective Procedure & Materials: Refer to Handout Data: Include group equations here; refer to WS for all Discussion: equations in their non-ionic form. Questions: Identify A-E and state reason. Then write full ionic Errors/Improvements Conclusion equation, cross off spectator ion, then write the net ionic equation for four of the reactions that occurred. Claim – given on handout; Evidence (signs of rxn-refer to table; 3 evidences) Science Concept (solubility, precipitate, ionic comp., aq) Lab Worksheet Lab #24 Write-Up (Due 3-6-12) Rubric Your Report (Worksheet) Conclusion Write a brief paragraph for your conclusion on worksheet and include: claim – given on WS evidence – make sure you refer to your observation table citing one reaction. science concept – link concepts of lab or class notes; evidence of chemical reactions; replacement reactions; formula writing, etc. Lab #23 Write-Up (Due 3-2-12) Rubric Your Report Lab Worksheet (Conservation During a Chemical Reaction) Table and Questions Lab #22 Write-Up (Due 2-21-12) Rubric Your Report Header (3-D Molecular Models) Objective Procedure & Materials: Refer to Handout Data: Table attached Discussion: Conclusion Questions #1-6; Errors/Improvements Claim – given on handout; Evidence (Tables- explain VSEPR and shape) Science Concept (hybridization; VSEPR models; polarity; intermolecular forces) Lab Worksheet Lab #21 Write-Up (Due 2-7-12) Rubric Your Report Lab Worksheet (Detecting Ions) Table and Questions Lab #20 Write-Up (Due 1-23-12) Rubric Your Report Lab Worksheet (Flame Test) Table and Questions Lab #19 Write-Up (Due 1-20-12) Rubric Your Report Header (Emission Spectroscopy) Objective Procedure & Materials: Refer to Handout Data: Spectra attached Calculations: Write neatly on lab handout (equations & units!) Discussion: Conclusion Questions; Errors/Improvements Claim – given on handout; Evidence (Spectra) Science Concept (electron energies, spectroscopy, continuous vs. line) Lab Worksheet Lab #16 Write-Up (Due 1-5-12) Rubric Your Report Header (Atomic/Ionic Radii Trends) Objective Procedure: How to make a Table & Graph in EXCEL. Data: Refer to attached tables and graphs. Discussion: Conclusion Questions (Type in report; include question) Errors/Improvements (omit) Claim – given on handout; Evidence (Graphs – state relationships and point to graphs as evidence of that observation.) Science Concept (ion formation; metals vs. nonmetals, etc.) Lab Worksheet Lab #14 Write-Up (Due 12-19-11) Rubric Your Report Header (Heat of Fusion of Ice) Objective Procedure & Materials: Refer to Handout Data : Calculations: Discussion: Neatly… in Lab Handout Questions (Refer to Lab WS #1-7) Errors/Improvements (question #1)) Conclusion Table & Graph: Temperature vs. Time Data Claim; Evidence (Data tables; cite examples of observations) Science Concept (calorimetry; phase changes; etc.) Lab Worksheet and Lab Data Sheet Lab #12 Write-Up (Due 12-7-11) Rubric Your Report Header (Dry Pressure of a Gas) Objective Procedure & Materials: Refer to Handout Data Illustration: Condition with meniscus above water in cylinder. Label diagram with ALL conditions or parameters Calculations: Discussion: Questions; Errors/Improvements Conclusion Show P (dry gas) and V @ STP calculations here Claim; Evidence (Data tables; cite examples of observations) Science Concept (Dalton’s Law Partial Pressure, Gas Laws, etc.) Lab Worksheet and Lab Data Tables Lab #11 Write-Up (Due 11-30-11) Rubric Your Report Header (Boyle’s Law) Objective Procedure & Materials: Refer to Handout Data: Refer to Tables in Handout Calculation for extra pressure in space provided on lab WS) Questions on back of Lab (2 sets) Errors/Improvements Conclusion (using total pressure and average volume) PxV Plot (using total pressure and average volume from Table 2 vs. trail number using a total of 8 trials even though only have data for 4.) Calculations: Discussion: Graphs: Volume vs. Pressure Claim; Evidence (Data tables; cite examples of observations) Science Concept (Inverse relationships, properties of gases, etc.) Lab Worksheet Lab #10 Write-Up (Due 11-18-11) Rubric Your Report Header (Percent Water in Hydrate) Objective Procedure and Materials: Refer to Handout Data: State Observations of hydrate before & after heating Summarize Data in table format Calculations: WS: % water in hydrate; % error calculation Discussion: Conclusion Questions on WS (neatly written!) Errors/Improvements Claim (given on handout) Evidence (Data tables; cite examples of observations) Science Concept (Hydrates: w/o color? Types; Uses – desiccants; crystal form) Lab Worksheet Lab #9 Write-Up Rubric Your Report Header (Effective Use of the Bunsen Burner) Objective Procedure and Materials: Refer to Handout Data: Organize your data in a table; Attach graph. Calculations: slope (question 3) Discussion: Questions (write on handout) Errors/Improvements (explain experimentally!) Conclusion* (Due 11-10-11) (Period 1 in class) Claim (given on handout) Evidence (Data table; cite examples) Science Concept (Rate definition; Combustion Reaction, etc.) Lab WS followed by Lab Data Sheet Lab #8 Write-Up Rubric Your Report Header (Measuring Specific Heat) Objective Procedure and Materials: Refer to Handout Data: Organize your data in a table. Calculations: Show equation, sub w/units; answer w/units. Include heat lost by metal, gained by water; specific heat calculation, % error (make sure to state heat lost metal = heat gained by water). Discussion: Questions (Write or restate questions) Errors/Improvements (explain experimentally!) Conclusion (Due 11-3-11) Claim (given on handout) Evidence (Data table; cite examples) Science Concept (Calorimetry, specific heat, conservation of energy) Lab WS followed by Lab Data Sheet Lab #7 Write-Up (Due 10-28-11) Rubric Your Report Header (Physical and Chemical Changes) Objective Procedure and Materials: Refer to Handout Data: Include OBSERVATION TABLE in report Discussion: State evidence for chemical reaction; describe physical changes Questions (Analysis); Errors/Improvements Conclusion Claim (given on handout) Evidence (Observation table; cite examples) Science Concept (Physical/Chemical changes; Evidence of chemical reactions; Dissolving as a physical change) Lab WS followed by Lab Data Sheet Lab #6 Activity (Due 10-20-11) Rubric Lab WS: Classifying Matter Completed Table Lab #5 Write-Up (Due 10-19-11) Rubric Your Report Header (Simulating Half-Life) Objective Procedure and Materials: Refer to Handout Data: Table attached. Graph: Average # Pennies vs. Half-Life Discussion: Conclusion Questions (WS); Errors/Improvements Claim (Half-Life can be simulated by probability) Evidence (Graph relationship; decay; no zero level) Science Concept (Define half-life; Extension – what isotope has the longest half-life? The shortest?) Lab WS followed by Lab Data Sheet Lab #4 Write-Up (Due 10-13-11) Rubric Your Report Header (Radioactive Decay Cards) Objective: Determine and illustrate the radioactive decay of U-238. Procedure & Materials: Refer to Handout Data Discussion: Type question then answer in report or answer by restating the question. Errors/Improvements Conclusion Table: List of Nuclear Equations Graph: Mass Number vs. Atomic Number Claim; Evidence (Refer to the graph for illustration of decay series) Science Concept (Cause and consequence of unstable nuclei; n/p ratio for stability; define radioactivity; describe radioactive particles and dangers; etc.) Lab Worksheet Lab #3 Write-Up (Due 10-5-11) Rubric Your Report Header (Avogrado’s Number) Objective Procedure and Materials: Refer to Handout Data: Refer to data table on Lab Worksheet Calculations: Calibration. Show work for Procedure III steps 3 and 4; highlight Discussion: final answer. Complete data entries on Lab WS using the full number and unit for each entry. Include % error using 6.0221415 x 1023 as the reference value. Conclusion Claim (given on handout) Evidence (Data table; cite examples; how was height of foil Science Concept (MOLE; Factor Label; units) Summarize how the diameter/side of the aluminum cell was obtained. Errors/Improvements determined; how was volume obtained?) Lab WS followed by Lab Data Sheet Lab #2 Write-Up (Due 9-27-11) Rubric Your Report Header (Atomic Mass of Beanium) Objective Procedure and Materials: Refer to Handout Data/Graph: See data table on worksheet Calculations: Average Atomic Mass: Method 1 vs. Method 2 Discussion: Conclusion Questions (WS); Errors and Improvements Claim; Evidence; Science Concept (meaning of “average” atomic mass; refer to calc. in table; define isotope; elements are mixtures; etc.) Lab WS followed by Lab Data Sheet Lab #1 Write-Up (Due 9-16-11) Rubric Your Report Header (Graphing Laboratory Data) Objective Procedure and Materials: Refer to Handout Data/Graph: See data table on worksheet; graph attached Calculations: Average; Slope; Percent Error (2.509g standard) Discussion: Conclusion Prelab questions (WS) Claim; Evidence; Science Concept labeling axes; significance of slope, etc.) (state importance of title, Lab WS followed by Lab Data Sheet Outlines for Lab Write-ups Honors Chemistry 2011-2012