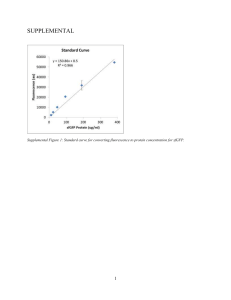
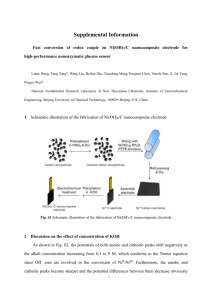
link to lecture - Welcome to brd4.braude.ac.il!
advertisement
Proceedings of FuelCell2006 Fourth International ASME Conference on Fuel Cell Science, Engineering and Technology Irvine, California. June 19-21 2006 FUELCELL2006-97031 ELECTRICAL CHARACTERIZATION OF A GLUCOSE-FUELED ALKALINE FUEL CELL 1Eugenia Bubis, 1Lea Mor, 1Nissim Sabag, 1Zeev Rubin, 1Ury Vaysban, 2Kas Hemmes, 1Pinchas Schechner 1Ort Braude College of Engineering, Karmiel, Israel. 2Delft University of Technology, Delft, The Netherlands. 1 Experimental Determination of: 1)Quasi-static polarization curves, V(J); 2) Power Density as a function of current density, PD(J) 3) Ohmic internal resistance of the cell, RFC. Experimental Conditions 1 [KOH] 2 [Glucose] 3 4 0.35 M 0.022 to 1.11 M. Room Temperature Sampling Time 0.1 s 2 Procedure FIGURE 2: FUEL CELL VOLTAGE AS A FUNCTION OF TIME DURING THREE CONSECUTIVE CONNECTION-DISCONNECTION CYCLES, [glu]0 = 0.67M; RL =10.29W., 9.28W, 8.4 W. 3 = V@(tc + 0.1s) = V@(td + 0.1s) FIGURE 3: FUEL CELL VOLTAGE AS FUNCTION OF TIME DURING A CONNECTION-DISCONNECTION CYCLE. RL = 10.29 W; [glu]0 = 0.67M 4 Polarization Curve for different [glu]0 VD JL RL A Small Values 5 Power Density as Function of Current Density of Various [glu]0 2 V PD(J) J L VD D RL A Low Values Maximun at 0.22 M Competence with non-electrochemical reactions 6 Internal Resistance Assuming that only the ohmic resistance can react immediately 1 - Voltage Divider Method Current at VIRC RL VIRC i c R L OCV R FC R L R FC R L (OCV VIRC ) VIRC 7 2 – Current Interrupt Method At the disconnection instant, there is a sudden increase in the cell’s voltage, VD, caused by the immediate annulment of the ohmic losses VD VIRD VD The current at the disconnection instant is: VD id RL RFC VD (VIRD VD )R L id VD 8 Horizontal continuous line indicates the RFCmean RFC as function of RL computed from the "VOLTAGE DIVIDER“ method [glu]0 = 0.22 and 0.67 M. 9 Internal Resistance as function of the glucose concentration RFCmean = RK + RC[glu]0 [W] Glucose Concentration dependent term Glucose Concentration independent constant RFC, 0.35 M KOH = 2.02 + 3.54[glu]0 [W] 10 Internal Resistance dependence on Glucose concentration, RC: RFCmean = RK + RC[glu]0 [W] The glucose concentration contributes to the ohmic resistance only in the volume between the two electrodes. As any ohmic resistor: RC c L [W·M-1] A AR C c 0.725R C W·M-1·m L Rc(1M glucose in 0.35 M KOH) = 2.56 W·m 11 Internal resistance factor independent of the glucose concentration, RK. RFCmean = RK + RC[glu]0 [W] RK includes ohmic resistances that don’t depend on glucose concentration, Rk = Rmetallic mesh + Rconnections + R0.35M KOH RK = 2.02 W > R0.35M KOH RK Contributes to the ohmic resistance only in the volume between the two electrodes. As any ohmic resistor R 0.35M KO H L 0.35M KO H A 1.38 0.35M KO H (1.38)(0.155) R0.35M KOH = 0.21 W < 2.02 W = RK 12 Conclusions At a [KOH] = 0.35M, the cell reaches peak performance at [glu]0 = 0.22M The two methods used to measure the internal resistance of the fuel cell, Voltage Divider and Current Interrupt, yield practically identical results Efforts will be directed towards development of practical glucose-fueled AFC, as electricity generators for portable devices Proposed Directions: • The effect of temperature on cell performance. • Development of nano-tube electro-catalytic electrodes 13