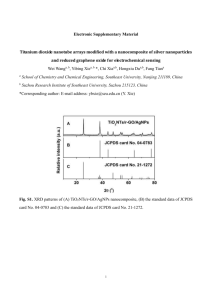
Wide range and excellent linearity detection of Glucose in
advertisement
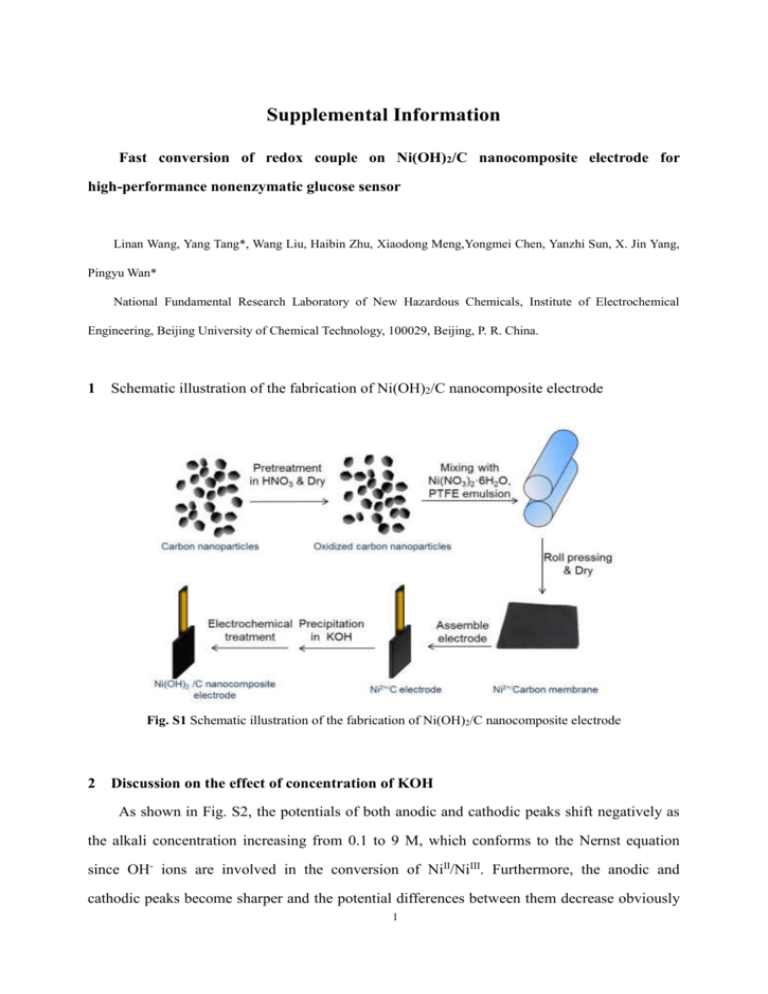
Supplemental Information Fast conversion of redox couple on Ni(OH)2/C nanocomposite electrode for high-performance nonenzymatic glucose sensor Linan Wang, Yang Tang*, Wang Liu, Haibin Zhu, Xiaodong Meng,Yongmei Chen, Yanzhi Sun, X. Jin Yang, Pingyu Wan* National Fundamental Research Laboratory of New Hazardous Chemicals, Institute of Electrochemical Engineering, Beijing University of Chemical Technology, 100029, Beijing, P. R. China. 1 Schematic illustration of the fabrication of Ni(OH)2/C nanocomposite electrode Fig. S1 Schematic illustration of the fabrication of Ni(OH)2/C nanocomposite electrode 2 Discussion on the effect of concentration of KOH As shown in Fig. S2, the potentials of both anodic and cathodic peaks shift negatively as the alkali concentration increasing from 0.1 to 9 M, which conforms to the Nernst equation since OH- ions are involved in the conversion of NiII/NiIII. Furthermore, the anodic and cathodic peaks become sharper and the potential differences between them decrease obviously 1 as the concentration of alkali increase, which means the high C OH- decreases the electrochemical polarization of NiII/NiIII. The current response (∆I) of the Ni(OH)2 /C nanocomposite electrode after 1 mM and 10 mM glucose additions with the concentration of OH- are showed in inset graph. ∆I 1 mM increases with the increase of COH before 1 M and decreases slightly when COH is above 1 M. However, the peak current response to 10 mM glucose (∆I 10 mM) is less than 8 times of ∆I 1 mM when COH is under 3 M. While in 7 M and 9 M KOH electrolyte, the ∆I 10 mM is about 9.5 times greater than ∆I 1 mM that means good linear response for glucose detection in wide range. This result is in agreement with the good linearity as shown in Fig 4D and Fig 6. The conversion rate of NiII/NiIII is high enough in the concentrated alkali electrolyte, and all the Ni(OH)2 that has been reduced by glucose can be converted into NiOOH immediately even in the presence of a large amount of glucose. That means the quantities of the NiOOH active sites almost keep constant, so the electro-catalytic oxidation ability is kept adequately and the sensitivity keep almost constant even the COHranging from μ M to mM. Considering the sensitivity and linear range, the optimal concentration of KOH is 1.0 M for obtaining the best sensitivity for glucose detection in this manuscript. Fig. S2. Cyclic voltammograms of the Ni(OH)2/C nanocomposite electrode at the scan rate of 100 mV/s in KOH solutions with concentrations (from right to left) of 0.1, 0.3, 0.5, 1, 3, 7, 9 M. Inset graph: the anodic peak current response (∆I) to 1 mM glucose and 10 mM glucose with the concentration of KOH. 2