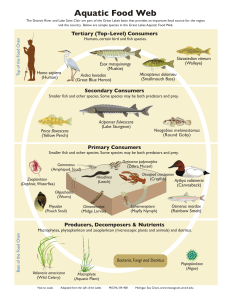
ENVI 21 Life in the Ocean
advertisement

Fig. 2.46 I. Zooplankton A. Holoplankton – – Spend entire lives as plankton Historically, epipelagic plankton moderately well sampled, especially within areas covered by commercial shipping lanes (CPR, LHPR) 1. Heterotrophic Protista – a. – – – – Among most important holoplanktonic grazers in terms of numbers and influence Dinoflagellates Heterotrophic or mixotrophic May reach 1 mm or more in size Feed on bacteria, diatoms, ciliates and other flagellates, either by using flagella to generate feeding currents or producing sticky cytoplasmic extensions that trap prey Ex - Noctiluca I. Zooplankton A. Holoplankton 1. Heterotrophic Protista b. Zooflagellates – Lack chloroplasts; strictly heterotrophic – Feed primarily on bacteria and detritus – Small (typically 2-5 μm in diameter) but may have high reproductive rates – Can become extremely abundant under favorable circumstances (20-80% of nanoplankton abundance by count) – May be important food source for larger secondary consumers I. Zooplankton A. Holoplankton 1. Heterotrophic Protista c. Foraminifera – Unicellular, amoeboid – Produce perforated calcareous tests typically composed of a series of chambers – Planktonic species range from ca. 30 μm to a few mm, smaller than benthic species – Capture food using slender pseudopodia (rhizopodia) that project through pores in test and trap small particles and organisms (bacteria, phytoplankton, small zooplankton) – Especially abundant in surface waters between 40oN and 40oS, and tests may form important components of calcareous sediments (foraminiferan oozes) – Ex - Globigerina Globigerinoides ruber I. Zooplankton A. Holoplankton 1. Heterotrophic Protista d. Radiolaria – Unicellular, ameboid – Similar to forams but tests composed of silica (SiO2) instead of CaCO3 – Range from ca. 50 μm to a few mm – Some species form gelatinous colonies up to 1 m across – Produce porous mineral tests through which branched pseudopodia (axopodia) are extended to feed on bacteria, other protists, phytoplankton (esp diatoms why??) and even small crustaceans – Common in all oceanic regions but especially abundant in cold waters, including deep sea – Sediments may consist of radiolarian oozes I. Zooplankton A. Holoplankton 1. Heterotrophic Protista e. Ciliophora – Present in all parts of ocean – May be extremely abundant in some areas – Cilia may be used for both locomotion and feeding – Typically prey on small phytoplankton, zooflagellates, small diatoms, bacteria – Tintinnids – ciliates with vase-shaped, proteinaceous external shells that aren’t found in sediments because of degradable nature – Relatively small (20-640 μm) but may be important because of wide distribution – Tintinnids feed primarily on nanoplanktonic diatoms and photosynthetic flagellates – May consume up to 60% of primary production in some coastal waters I. Zooplankton A. Holoplankton 2. Cnidaria – Includes medusae and siphonophores – Medusae range from a few mm to 2 m across (Tentacles of Cyanea capillata may be 30-60 m long) and feed using tentacles with cnidocytes/nematocysts – Siphonophores are colonial cnidarians; individuals perform specialized functions (e.g. swimming, feeding, reproduction) that benefit colony – Ex - Portugese man-of-war (Physalia); portion floats on sea surface and tentacles may extend 10 m into water – Siphonophores may reach 50-70 m in length – Feed primarily on zooplankton and appropriately-sized nekton Cyanea capillata I. Zooplankton A. Holoplankton 3. Ctenophora – Carnivorous: eat fish eggs and larvae as well as smaller zooplankton – Feed using paired, sticky tentacles (tentaculate) or large, ciliated oral lobes (lobate) – May be ecologically significant as competitors for food resources – Populations may increase explosively at certain times of year in certain areas Pleurobrachia Tentaculate Beroe Lobate I. Zooplankton A. Holoplankton 4. Chaetognatha – Among the most abundant carnivorous plankton, worldwide – Exclusively marine and found over a wide depth range – Relatively small (max. length ca. 4 cm) but voracious predators – Sit-and-wait predators – Primary food item = small zooplankton I. Zooplankton A. Holoplankton 5. Annelida – Relatively few known holoplanktonic annelids, all in class Polychaeta – Planktonic polychaetes present throughout ocean – Prey most frequently on small zooplankton – Typically small (up to 20 cm); some may be bigger I. Zooplankton A. Holoplankton 6. Mollusca – Relatively few holoplanktonic mollusks – Ex - Janthina a. Heteropoda – Small group closely related to snails – Swim by undulating fin (modified gastropod foot) – Some species have a small calcium carbonate shell into which a portion of body can withdraw defensively; lost in many species – Visual predators on planktonic molluscs, copepods, chaetognaths, salps and siphonophores – Well-developed eyes – Most common in tropical waters I. Zooplankton A. Holoplankton 6. Mollusca b. Pteropoda – Two forms: thecate (thecosome – shelled) and athecate (gymnosome - no shell) – Thecate forms have calcareous shells that may be coiled or cup-shaped. – Thecosomes swim using paired “wings” (modified gastropod foot) – Thecosomes suspension feeders, trapping particles using large mucus webs – Typical diet includes phytoplankton, small zooplankton and detrital material – Some thecosomes may be important food items for pelagic fishes, including some commercially important species (e.g. herring, etc.). – Shells of thecate pteropods may accumulate in sediments (pteropod oozes) – Gymnosomes typically predatory, often feeding on other pteropods – May get quite large (to 8.5 cm) and are common throughout the oceans I. Zooplankton A. Holoplankton 7. Arthropoda – Major group = subphylum Crustacea a. Copepoda – Predominant class of holoplanktonic crustaceans is the Copepoda – Calanoida – Most common group of copepods with nearly 2000 described species – Present throughout ocean and comprise a major proportion of planktonic biomass in many areas – Typically small (< 6 mm) though some large species may exceed 1 cm – Most are primary consumers, feeding on phytoplankton – Some may be carnivorous on small zooplankton – Development involves 12 different stages, 6 naupliar stages (NI - NVI) and 6 copepodite (CI - CVI) stages, last of which is mature adult Herbivorous vs. Predatory Copepod Copepod Suspension Feeding Mechanism Selective Particle Sorting Calanoid I. Zooplankton A. Holoplankton 7. Arthropoda – Major group = subphylum Crustacea a. Copepoda – Predominant class of holoplanktonic crustaceans is the Copepoda – Calanoida – Most common group of copepods with nearly 2000 described species – Present throughout ocean and comprise a major proportion of planktonic biomass in many areas – Typically small (< 6 mm) though some large species may exceed 1 cm – Most are primary consumers, feeding on phytoplankton – Some may be carnivorous on small zooplankton – Development involves 12 different stages, 6 naupliar stages (NI - NVI) and 6 copepodite (CI - CVI) stages, last of which is mature adult Fig. 2.7 I. Zooplankton A. Holoplankton 7. Arthropoda a. Copepoda – Cyclopoida – Differ from calanoids: shorter antennae (used by some species to capture prey), more segments in abdomen – Over 1000 species but most are benthic – About 250 planktonic species and some (e.g. Oithona) may be abundant locally Calanoid I. Zooplankton A. Holoplankton 7. Arthropoda a. Copepoda – Harpacticoida – Predominantly benthic – Typically small – Seldom important elements of zooplankton I. Zooplankton A. Holoplankton 7. Arthropoda b. Euphausiacea (Krill) – Shrimp-like organisms typically 15-20 mm long but exceeding 10 cm in some species – Generally omnivorous; may consume both plant and animal material but prefer phytoplankton and phytoplankton detritus when available – May be extremely important ecologically: Keystone species in Southern Ocean = E. superba – May be very abundant, e.g. Euphausia superba “super-swarms” in the Southern Ocean have been estimated at 450 sq km x 200 m @ >1000 m-3 – Typically very mobile, and most net-based surveys may underestimate abundance recent switch to use of acoustic techniques for surveys I. Zooplankton A. Holoplankton 7. Arthropoda c. Amphipoda – Typically small animals, though some species may exceed 10 cm – Planktonic forms typically free-living carnivores, but some species live in close association with salps, medusae and other gelatinous zooplankton – Typically constitute a minor component of zooplankton, gravimetrically – Unlike most planktonic crustaceans, amphipods brood their young I. Zooplankton A. Holoplankton 7. Arthropoda d. Ostracoda – Typically minor components of zooplankton community – Most species quite small (few mm), though Gigantocypris can exceed 2 cm in diameter – Some important as food sources for other species, notably small fishes I. Zooplankton A. Holoplankton 7. Arthropoda e. Mysidacea – Closely related to amphipods – Seldom important components of planktonic communities – Some species are diel vertical migrators and important food items for certain species (e.g. fishes living on shallow banks) I. Zooplankton A. Holoplankton 7. Arthropoda f. Decapoda – Among largest zooplankton: May reach 10+ cm – Many species are diel vertical migrators and often exhibit net avoidance – Often omnivores or predators, feeding primarily on smaller planktonic crustaceans (e.g. copepods, euphausiids) I. Zooplankton A. Holoplankton 8. Chordata a. Appendicularians/Larvaceans – Closely related to sea squirts – Referred to as Larvaceans because of resemblance to tadpole larvae of sea squirts – Most species produce spherical mucus houses – Typical larvacean bodies are a few mm long; houses may reach a meter in diameter – Movements of animal’s tail pump water through house across a series of mucus mesh filters that strain particles from water – Link – Periodically, filters become clogged and larvacean abandons house and builds a new one; takes a few minutes and may be repeated more than 10 times a day – Larvaceans grow rapidly, may have generation times of only a few weeks and are among the most abundant zooplankton in some coastal regions (e.g. up to 5000 m-3 in Monterey Bay) – Abandoned larvacean houses may be important components of marine snow in some areas I. Zooplankton A. Holoplankton 8. Chordata b. Thaliacea (Salps) – Common in near-surface waters, though some deepliving forms – Swim using radial bands of muscle to pump water through central body cavity – Same stream of water passed through mucus net that filters out food particles – Food particles consist primarily of bacteria and phytoplankton, ranging from 1 μm to 1 mm – May “bloom” to form dense aggregations – High abundance and high feeding rates may reduce abundance of small particles/organisms in water column and effectively outcompete other consumers for food resources (e.g. krill in Southern Ocean) I. Zooplankton B. Meroplankton – – – – – – – Meroplankton spend portion of life in plankton; adult stage typically non-planktonic About 70% of benthic marine species have a planktonic stage in their life cycle Planktonic stage of a benthic organism’s life may last minutes to months Presence of particular species in meroplankton typically related to spawning events, often in response to environmental cues (e.g. warmer temperatures in temperate latitudes, rainfall or lunar cycles in tropical waters) Important component of meroplankton is ichthyoplankton, fish eggs and larvae Some fish eggs may be extremely abundant (e.g. 4 x 1014 pilchard eggs in English Channel) and energetically important as food sources for other pelagic organisms Marine organisms with pelagic larvae exhibit two basic strategies for nourishing larval stages Fig. 2.25 I. Zooplankton B. Meroplankton – – – – – – – Meroplankton spend portion of life in plankton; adult stage typically non-planktonic About 70% of benthic marine species have a planktonic stage in their life cycle Planktonic stage of a benthic organism’s life may last minutes to months Presence of particular species in meroplankton typically related to spawning events, often in response to environmental cues (e.g. warmer temperatures in temperate latitudes, rainfall or lunar cycles in tropical waters) Important component of meroplankton is ichthyoplankton, fish eggs and larvae Some fish eggs may be extremely abundant (e.g. 4 x 1014 pilchard eggs in English Channel) and energetically important as food sources for other pelagic organisms Marine organisms with pelagic larvae exhibit two basic strategies for nourishing larval stages I. Zooplankton B. Meroplankton 1. Planktotrophic – Eggs relatively small and contain little stored energy – Species with planktotrophic development have higher fecundities than species with lecithotrophic development (e.g. plaice - 250,000 eggs, haddock - 500,000 eggs, cod - >1,000,000 eggs) – Low per-egg energy investment lower per-egg survivorship but vastly greater numbers of propagules for a given reproductive energy investment – Survivorship typically very low (e.g. early life mortality in cod estimated at around 99.999%). – Planktotrophic larvae feed in plankton, typically have long larval life spans, and may travel very long distances (teleplanic larvae - e.g. coral planula larvae) I. Zooplankton B. Meroplankton 2. Lecithotrophic – Eggs relatively large and contain substantial stored energy – Species with lecithotrophic development have lower fecundities than species with planktotrophic development (typically <1000) – High per-egg energy investment higher per-egg survivorship but fewer propagules for a given reproductive energy investment – Yolk sac typically used to sustain larva while mouth and gut finish developing – Lecithotrophic larvae typically do not feed in plankton (though many do), have short larval life spans (generally less than a week and sometimes a few hours), and generally don’t disperse very long distances – Often lecithotrophic eggs are buoyant and species exhibit ontogenetic vertical migration within water column (e.g. Sebastolobus altivelis) I. Zooplankton C. Vertical Distribution 1. Planktocline – In stable water columns with very shallow mixed layers, e.g. at low latitudes in eastern parts of oceans or mid-latitudes toward end of summer, zooplankton abundance may be much higher in mixed layer than below it, with highest abundances just above thermocline – Abundance typically declines sharply near bottom of thermocline = planktocline – Some controversy: Does zone of maximum zooplankton biomass coincides with region of maximum phytoplankton biomass or productivity? – Recent evidence: macrozooplankton feed at or near productivity maximum; microzooplankton feed at or near phytoplankton biomass maximum I. Zooplankton C. Vertical Distribution 2. Diel Vertical Migration (DVM) a. Patterns 1) Nocturnal – Surface at night, depth during day 2) Twilight – Sunset ascent, midnight sink, dawn descent 3) Reverse – Surface during day, depth at night b. Nature – Different species and life stages exhibit different vertical migration patterns and depth ranges – Major trigger: Light – Solar eclipse Premature migration I. Zooplankton C. Vertical Distribution 2. Diel Vertical Migration (DVM) c. Value 1) Access to food in surface waters at night with reduced vulnerability to visual predators – Daytime depths of predators not dark enough to prevent predation – Some zooplankton migrate deeper than necessary to avoid high predation – Many predators also migrate – Tested experimentally in a limited way by studying DVM in response to different predation pressures – Ohman (1990): Pseudocalanus newmani undergoes migration, reverse migration or no migration when major predators are visually hunting planktivorous fishes, nocturnally feeding nonvisual zooplankton, or absent I. Zooplankton C. Vertical Distribution 2. Diel c. 2) – – – 3) – – d. – – Vertical Migration (DVM) Value Energetic benefits Descending into cooler waters during day reduces metabolic rates and makes more efficient use of food Support: DVM less common in polar waters Question: Do energetic benefits exceed costs of migration? Replenishment of food supply No conclusive evidence Low food may enhance or suppress DVM Consequences Mixing of populations enhances gene flow Active transport of organic material to sea floor through trophic ladder I. Zooplankton C. Vertical Distribution 3. Seasonal Vertical Migration – Seasonal patterns in vertical distribution relatively common among species in temperate and polar regions as well as upwelling zones, but generally not in tropical species Fig. 2.42 Fig. 2.43 I. Zooplankton D. Horizontal Distribution – 1. Wide range of spatial scales Water Mass Affiliations – Cosmopolitan species have wide or even global distributions – Other species are local or closely associated with a particular set of hydrographic conditions – Some highly specific species can be indicators for a particular water mass – Concept of indicator species most commonly applied to foraminifera, copepods and chaetognaths (sufficiently abundant) – Ex: Omori (1965) used distributions of copepod species assemblages to identify three major oceanic regions in North Pacific: a) Cold offshore region characterized by Neocalanus plumchrus and Calanus cristatus b) Warm offshore region characterized by Calanus pacificus c) Neritic region characterized by Pseudocalanus minutus and Acartia longiremis I. Zooplankton D. Horizontal Distribution 1. Latitudinal Patterns – Strong N-S temperature gradient distributional affinities related to water temperature – About 50% of all epipelagic zooplankton spp. have distributional centers in tropical and subtropical waters with some presence in temperate waters – About one-third of epipelagic holoplankton are restricted to tropical and subtropical waters – Other species restricted to cold waters at high latitudes – Some species endemic to either Arctic or Antarctic I. Zooplankton D. Horizontal Distribution 1. Latitudinal Patterns – Some species have bipolar distribution – Ex: Pteropods - Limacina helicina and L. retroversa, Amphipod - Parathemisto gaudichaudii, Siphonophore - Dimophyes arctica – Arctic-Antarctic species pairs have bipolar distributions and occupy similar niches within communities at both poles – Ex: Gymnosome pteropods, Clione limacina (Northern Hemisphere) and C. antarctica (Southern Hemisphere), are morphologically similar and both feed on two Limacina species – Bipolarity may have arisen through a) Polar emergence b) Relict distributions – General trend toward decreasing species diversity with latitude – Groups that occur at low and high latitudes typically have fewer high-latitude species, while some groups (e.g. heteropod mollusks) have no high-latitude representatives – Many “circumglobal tropical-subtropical” species occur in warm waters of Atlantic, Pacific and Indian oceans (e.g. Janthina, Glaucus, some euphausiids, chaetognaths and amphipods) – Tethyan Distribution