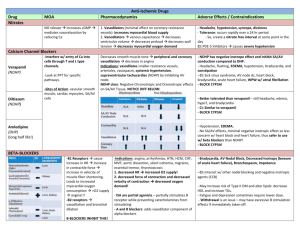
TITLE: Reduction in Platelet Reactivity With Prasugrel 5 mg in Low
advertisement
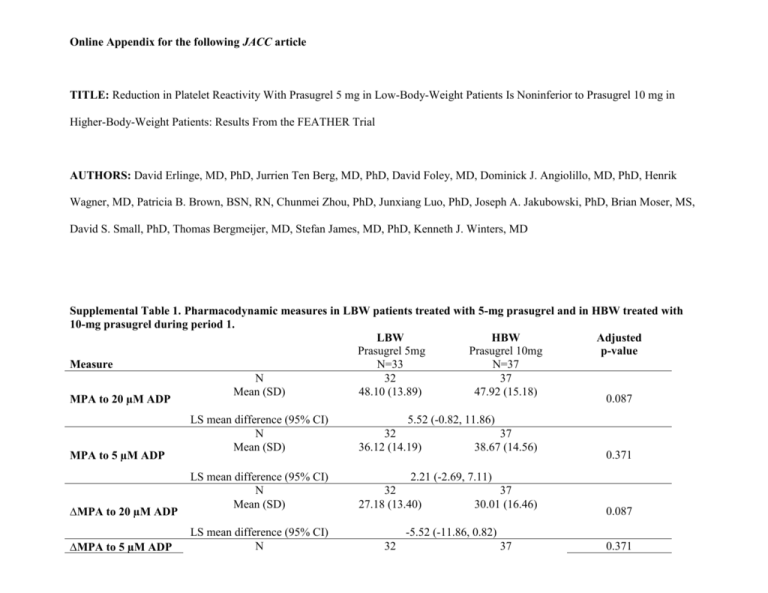
Online Appendix for the following JACC article TITLE: Reduction in Platelet Reactivity With Prasugrel 5 mg in Low-Body-Weight Patients Is Noninferior to Prasugrel 10 mg in Higher-Body-Weight Patients: Results From the FEATHER Trial AUTHORS: David Erlinge, MD, PhD, Jurrien Ten Berg, MD, PhD, David Foley, MD, Dominick J. Angiolillo, MD, PhD, Henrik Wagner, MD, Patricia B. Brown, BSN, RN, Chunmei Zhou, PhD, Junxiang Luo, PhD, Joseph A. Jakubowski, PhD, Brian Moser, MS, David S. Small, PhD, Thomas Bergmeijer, MD, Stefan James, MD, PhD, Kenneth J. Winters, MD Supplemental Table 1. Pharmacodynamic measures in LBW patients treated with 5-mg prasugrel and in HBW treated with 10-mg prasugrel during period 1. LBW HBW Adjusted Prasugrel 5mg Prasugrel 10mg p-value N=33 N=37 Measure N 32 37 Mean (SD) 48.10 (13.89) 47.92 (15.18) 0.087 MPA to 20 µM ADP MPA to 5 µM ADP ∆MPA to 20 µM ADP ∆MPA to 5 µM ADP LS mean difference (95% CI) N Mean (SD) 5.52 (-0.82, 11.86) 32 37 36.12 (14.19) 38.67 (14.56) LS mean difference (95% CI) N Mean (SD) 2.21 (-2.69, 7.11) 32 27.18 (13.40) LS mean difference (95% CI) N 32 37 30.01 (16.46) 0.371 0.087 -5.52 (-11.86, 0.82) 37 0.371 Mean (SD) IPA to 20 µM ADP IPA to 5 µM ADP RPA to 20 µM ADP RPA to 5 µM ADP IRPA to 20 µM ADP IRPA to 5 µM ADP VN-P2Y12 (device reported %) VN-P2Y12 (PRU) VASP PRI 31.15 (10.47) 33.90 (9.88) LS mean difference (95% CI) N Mean (SD) -2.21 (-7.11, 2.69) 32 36.00 (17.09) LS mean difference (95% CI) N Mean (SD) -6.91 (-16.16, 2.33) 32 37 47.37 (17.34) 47.81 (14.27) LS mean difference (95% CI) N Mean (SD) -3.63 (-10.84, 3.57) 31 28.51 (17.30) 37 37.28 (24.69) 37 29.11 (21.66) LS mean difference (95% CI) N Mean (SD) 31 13.75 (13.08) LS mean difference (95% CI) N Mean (SD) 0.31 (-5.11, 5.73) 31 37 61.63 (22.60) 61.99 (31.46) LS mean difference (95% CI) N Mean (SD) -8.21 (-21.04, 4.62) LS mean difference (95% CI) N Mean (SD) 0.140 0.317 0.220 5.55 (-3.41, 14.52) 31 79.48 (18.25) 37 18.11 (16.38) 37 75.82 (18.99) 0.910 0.205 0.772 -1.12 (-8.80, 6.56) 32 58.9 (19.57) 34 67.1 (22.07) LS mean difference (95% CI) N Mean (SD) 32 129.53 (62.14) LS mean difference (95% CI) N Mean (SD) 28.64 (-0.31, 57.59) 32 36 33.54 (15.77) 27.79 (18.13) 0.075 -8.80 (-18.52, 0.92) 34 102.09 (69.49) 0.052 0.196 LS mean difference (95% CI) 5.49 (-2.91, 13.89) Abbreviations: ADP=adenosine diphosphate; CI=confidence interval; HBW=higher body weight; IPA= inhibition of platelet aggregation; IRPA= inhibition of residual platelet aggregation; LBW=low body weight; LS=least squares; MPA=maximum platelet aggregation; PRI=platelet reactivity index; PRU=P2Y12 reaction units; RPA= residual platelet aggregation; VN-P2Y12=VerifyNow® P2Y12; VASP=vasodilator-associated phosphoprotein. Supplemental Table 2. LBW HBW Prasugrel 5 mg Prasugrel 10 mg N=33 N=37 MPA to 20µM ADP >50%, n (%) 14 (43.8) 14 (37.8) 0.633 IPA to 20 µM ADP <20%, n (%) 6 (18.8) 6 (16.2) 1.000 RPA 5 µM ADP >14%, n (%) 9 (29.0) 18 (48.6) 0.137 VN-P2Y12 PRU >235, n (%) 1 (3.1) 1 (2.9) 1.000 VN-P2Y12 <15%, n (%) 2 (6.3) 1 (2.9) 0.608 VASP-PRI ≥50%, n (%) 4 (12.5) 5 (13.9) 1.000 p-value Abbreviations: ADP=adenosine diphosphate; HBW=high body weight; IPA=inhibition of platelet aggregation; LBW=low body weight; MPA=maximum platelet aggregation; PRI=platelet reactivity index; PRU=P2Y12 reaction units; RPA=residual platelet aggregation. Supplemental Methods Trial Design At screening (Visit 1), patients were weighed and categorized as either low body weight (<60 kg, LBW) or higher body weight (>60 kg, HBW). At the end of the aspirin run-in phase, patients in each cohort were randomized into two treatment sequences on a continued background of aspirin. All LBW patients received prasugrel 5-mg during Period 1, were subsequently randomized to either prasugrel 10-mg or clopidogrel 75-mg for Period 2, then were crossed over to the alternate study dose of either clopidogrel 75-mg or prasugrel 10-mg for Period 3. All HBW patients received prasugrel 10-mg for Period 1, received either prasugrel 5-mg or clopidogrel 75-mg for Period 2, and were crossed over to the alternate treatment for Period 3. Period 1 was conducted in a single-blind fashion (only patients were blinded to treatment) to allow study investigators to monitor initial tolerability to treatment; Periods 2 and 3 were conducted double-blind. Before proceeding to Period 2, LBW patients needed to have tolerated the 5-mg prasugrel dose in Period 1 (with tolerability defined as an absence of a study drugrelated adverse event that resulted in interruption and/or discontinuation of study drug, per investigator opinion). Daily study treatment in Periods 1, 2, and 3 consisted of three tablets, one active treatment (prasugrel 5-mg, prasugrel 10-mg, or clopidogrel 75-mg), and two placebos to match the other two study drugs. The study was performed at 5 clinical sites in 4 countries (Ireland, the Netherlands, Sweden, and USA) from March 2010 to August 2011. Participants Key trial exclusion criteria included unstable CAD (defined as newly diagnosed, increased, or resting angina; hospitalization for unstable angina or myocardial infarction within the prior 30 days; or percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) within the prior 90 days), a history of refractory ventricular arrhythmias within the last 6 months or prior implantation of a defibrillator device, congestive heart failure of New York Heart Association class III or IV within 6 months prior to screening, uncontrolled hypertension (systolic blood pressure >180 mm Hg or diastolic blood pressure >110 mm Hg at screening), planned coronary revascularization or other surgical procedures planned within 60 days following randomization, or a prior history or presence of significant bleeding disorders, abnormal bleeding tendency, or coagulation disorders. Additionally, patients could not be enrolled if they had used antiplatelet agents (excluding aspirin) within 10 days prior to screening, had used (or planned to use) heparin, oral anticoagulants, or fibrinolytic agents within 30 days of screening, or were being treated with nonsteroidal anti-inflammatory drugs or cyclooxygenase-2 inhibitors that could not be discontinued for the duration of the study. Study Objectives and Measures The primary objective of FEATHER was to compare MPA responses to 20 µM adenosine diphosphate (ADP) by light transmission aggregometry (LTA) in LBW patients receiving prasugrel 5-mg versus HBW patients receiving prasugrel 10-mg. Secondary pharmacodynamic (PD) measures included MPA response to 5µM ADP, residual platelet aggregation (RPA), inhibition of platelet aggregation (IPA), P2Y12 reaction units (PRU) as assessed by the VerifyNow® P2Y12 (VN-P2Y12) assay, and vasodilator-associated stimulated phosphoprotein (VASP) phosphorylation levels. VASP phosphorylation levels were expressed as the platelet reactivity index, PRI. For LTA and VASP, samples were collected into citrate blood collection tubes at baseline and during the predose trough at the end of Periods 1, 2 and 3 (Day 12 ± 2). Citrated Greiner™ tubes were used for sample collection for the VN-P2Y12 assay at the same time points. LTA (without adjustment of platelet count), VN-P2Y12, and VASP assays were conducted as described in previous publications (1,2). Plasma samples for pharmacokinetic (PK) analysis were collected at 0.5, 1, 2, 3, and 4 hours following the first dose of each period and the last dose of Period 3, and prasugrel’s and clopidogrel’s active metabolite concentrations were assayed as described in previous publications (3,4). Resulting data were analyzed noncompartmentally using the log-linear trapezoidal method of WinNonlin version 5.3, Model 200 (Pharsight, Cary, NC). The primary parameter for analysis was the area under the concentration–time curve calculated through the last quantifiable concentration up to 4 hours postdose, AUC(0-tlast). Safety data included the recording of adverse events, clinical laboratory evaluations, measurements of vital signs, electrocardiograms, and physical examinations. Statistical Analysis The primary non-inferiority analysis compared the median of MPA to 20 µM ADP for prasugrel 5-mg in LBW patients to the 75th percentile of the MPA for prasugrel 10-mg in HBW patients at the end of Period 1. The upper limit of the one-sided 97.5% confidence interval (CI) of the difference was estimated from resampling data with replacement through bootstrap methodology and was compared with the pre-specified margin of 15 percentage points. Based on the LTA data from four previous studies of prasugrel in aspirin-treated patients with CAD (5,6,7,8), we assumed an 8 percentage point mean difference of MPA to 20 µM ADP between prasugrel 5-mg in LBW patients and prasugrel 10-mg in HBW patients, an SD of 18% for prasugrel 5-mg in LBW patients, and an SD of 12% for prasugrel 10-mg in HBW patients; under these assumptions, having data for the primary endpoint on 33 patients in each arm provides >80% power for the non-inferiority test of the primary endpoint at a one-sided 2.5% significance level. Pre-specified HPR thresholds were: MPA to 20 μM ADP >50% (9); inhibition of platelet aggregation (IPA) to 20 μM ADP <20% (10) ; RPA to 5 μM ADP >14% (11); VN-P2Y12 PRU>235 (12); VN-P2Y12, device-reported inhibition <15% (13); VASP based PRI ≥50% (14). Within each BW cohort, the comparisons between treatment groups on PD response were performed using a mixed-effect model with repeated measure analysis on PD response after 12 ± 2 days of daily dosing were performed by a mixed-effect model with repeated measure analysis with treatment (prasugrel 5-mg, prasugrel 10-mg and clopidogrel 75-mg), study site, period, treatment sequence and the baseline value as the fixed effects, and patient as a random effect. Within each BW cohort, rate of HPR was compared between treatment groups using a generalized linear model with repeated measures, through generalized estimating equations. All statistical analyses were performed using SAS version 9.1 (Cary, North Carolina USA). 1. Jakubowski JA, Payne CD, Li YG, Brandt JT, Small DS, Farid NA, Salazar DE, Winters KJ. The use of the VerifyNow P2Y12 point-of-care device to monitor platelet function across a range of P2Y12 inhibition levels following prasugrel and clopidogrel administration. Thromb Haemost 2008: 99(2):409-15 2. Jakubowski JA, Bourguet N, Boulay-Moine D, et al. Comparison of a new ELISA assay with the flow cytometric assay for platelet vasodilator-associated stimulated phosphoprotein (VASP) phosphorylation in whole blood to assess P2Y12 inhibition. Thromb Haemost 2011: 107(2) 3. Farid NA, McIntosh M, Garofolo F, et al. Determination of the active and inactive metabolites of prasugrel in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2007; 21(2):169-179. 4. Takahashi M, Pang H, Kawabata K, et al. Quantitative determination of clopidogrel active metabolite in human plasma by LC-MS/MS. J Pharm Biomed Anal 2008; 48:1219-1224. 5. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27(10):1166-1173. 6. Wallentin L, Varenhorst C, James S, et al. Prasugrel achieves greater and faster P2Y12 receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J 2008; 29(1):21-30. 7. Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and AggregationThrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116(25):2923-2932. 8. Montalescot G, Sideris G, Cohen R, et al. Prasugrel compared with high-dose clopidogrel in acute coronary syndrome. Thromb Haemost 2010; 103(1):213-223. 9. Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007; 115(6):708-716 10. Weerakkody GJ, Brandt JT, Payne CD, et al. Clopidogrel poor responders: an objective definition based on Bayesian classification. Platelets 2007; 18(6):428-435. 11. Hochholzer W, Trenk D, Bestehorn H-P, et al. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol 2006; 48(9):1742-1750. 12. Price MJ, Endemann S, Gollapudi RR, Valencia R, et al. Prognostic significance of postclopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drugeluting stent implantation. European Heart Journal 2008; 29(8):992–1000. 13. Cuisset T, Hamilos M, Sarma J, et al. Relation of low response to clopidogrel assessed with point-of-care assay to periprocedural myonecrosis in patients undergoing elective coronary stenting for stable angina pectoris. Am J Cardiol 2008; 15(12):1700-1703 14. Barragan P, Bouvier JL, Roquebert PO, et al. Resistance to thienopyridines: clinical detection of coronary stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Cathet Cardiovasc Intervent 2003; 59:295-302.