IRB: Documentation and General Requirements of Assent
advertisement

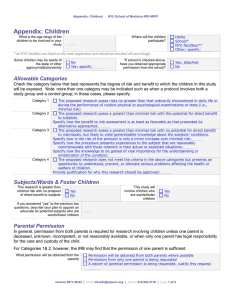
IRB: Documentation and General Requirements of Assent Policy number: 704 A References: 45 CFR 46.117 21 CFR 50.27 http://www.research.umn.edu/irb/forms.html AAHRPP II.3.F and G AAHRPP II.4.B Date: 05/19/14 Policy Owner: Executive Director, HRPP Cross References: 704 Assent of Subjects Appendix Y Definitions: None 1.0 Reason for Policy The reason for this policy is to describe the process for documentation of assent. 2.0 Scope of Policy The scope of this policy is the University community and its healthcare components. 3.0 Policy Statement The investigator is expected to create an assent form that is age-appropriate and study-specific, taking into account the typical child's experience and level of understanding, and composing a document that treats the child respectfully and conveys the essential information about the study. The form should: Tell why the study is being conducted Describe what will happen and for how long or how often Indicate that it is up to the child to participate and that it's okay to say no Explain if it will hurt and for how long and how often Indicate what the child's other choices are Describe any good things that might happen Page 1 of 2 Indicate whether there is any compensation for participating Ask for questions The document should be limited to one page where possible. Illustrations might be helpful, and larger type makes a form easier for younger children to read. Studies involving older children or adolescents should include more information and may use more complex language. Parental consent forms also may be revised to include the assent of older children, provided the directive language is revised and the appropriate signature and date lines are added. Information Sheets In circumstances where the research involves an option for a child whose illness has not responded to other available treatments or for whom standard therapies are not suitable, it is unlikely that a child’s refusal to participate would be honored. In such cases, it is more appropriate to provide children with an information sheet that contains the same information as an assent form would, but without the indication of choice. This respects the child’s right to understand the research and what will happen to him/her, while acknowledging that there are situations in which a parent’s authority must override the wishes of the child. 4.0 Required approvals for this document Title Executive Director, HRPP 5.0 Revision History Revision 05/19/14 11/09/09 06/22/09 09/26/06 Reason for change AAHRPP revision Update AAHRPP references Reformat Policy Development Date of release 09/02/14 11/09/09 06/22/09 To obtain a copy of a historical policy, e-mail IRB at irb@umn.edu or call 612-626-5654 Page 2 of 2