Topic 3: The Chemistry of Life
advertisement

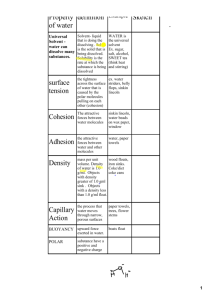
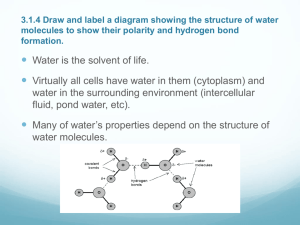
Organic Chemistry is the chemistry of carbon compound Biochemistry is the branch of organic chemistry which attempts to explain the chemistry in living organisms Four main types of organic molecule Carbohydrates Lipids Proteins Nucleic acids Assessment statements: 3.1.1 states that the most frequently occurring chemical elements in living things are carbon, hydrogen, oxygen and nitrogen 3.1.2 state that a variety of other elements are needed by living organisms, including sulfur, calcium, phosphorus, iron, and sodium 3.1.3 state one role for each of the elements mentioned in 3.1.2 3.1.4 draw and label water molecules to show their polarity and hydrogen bond formation 3.1.5 outline the thermal, cohesive, and solvent properties of water 3.1.6 explain the relationship between the properties of water and its uses in living organisms as a coolant, medium for metabolic reactions and transport medium Four most common elements found in living things Carbon, hydrogen, oxygen and nitrogen These elements are used in the molecular structures of all carbohydrates, proteins, lipids and nucleic acids Element Example role in plants Example role in animals Example role in prokaryotic Sulfur In some amino acids In some amino acids In some amino acids Calcium Co-factor in some enzymes Co-factor in some enzymes and component of bones Co-factor in some enzymes Phosphorus Phosphate group in ATP Phosphate group in ATP Phosphate group in ATP Iron In cytochromes In cytochromes and in heamoglobin In cytochromes Sodium In membrane function In membrane function and sending nerve impulses In membrane function Water is the solvent of life Virtually all cells have water within (cytoplasm) and water in the surrounding environment (intercellular fluid, pond water, etc) Water is an incredibly abundant substances on Earth and has very interesting properties Many of these properties depend on the structure of water molecules H H The hydrogen and oxygen atoms in a single water molecule are held together by a type of bond called a polar covalent bond Polar covalent bond results from an unequal sharing of electrons Results in a slight negative charge at the oxygen end and a slight positive charge at the end of the two hydrogen Because the two ends of each water molecule have opposite charges, water molecules interact with each other Thermal Properties High specific heat This means that water can absorb or give off a great deal of heat without changing temperature greatly High heat of vaporization This means that water absorbs a great deal of heat when it evaporates Cohesive Properties Water molecules are highly cohesive Is when molecules of the same type are attracted to each other The attraction is due to the polar covalent bonding When water cools below the freezing point, molecular motion has slowed to the point where these polar attractions become locked into place and an ice crystal forms This cohesion between liquid water molecules explains a variety of events, including Why water forms into droplets when spilled Why water has a surface tension that allows some organisms to ‘walk on water’ How water is able to move as a column in the vascular tissues of plants Why water has a high heat capacity and high heat of vaporization Solvent Properties Water is an excellent solvent of other polar molecules ‘like dissolves like’ Most molecules typically found inside and outside of most cells are also polar molecules Most types of lipids are relatively non-polar Aqueous solution Location Common reactions Cytoplasm Fluid inside cells but outside organelles Glycolysis/protein synthesis reactions Nucleoplasm Fluid inside nuclear membrane DNA replication/transcription Stroma Fluid inside chloroplast membrane Light-dependent reactions of photosynthesis Blood plasma Fluid in arteries, veins and capillaries Loading and unloading of respiratory gases/clotting Examples of eater as a solvent in plants and animals Properties of eater makes it an excellent medium for transport Vascular tissue in plants carries water and a variety of dissolved substances Xylem carries water and dissolved minerals up from the root system to the leaves of a plant Phloem then transports dissolved sugars from the leaves to the stems, roots, and flowers of a plant Blood is the most common transport medium in animals and is largely made up of water Transport medium for red blood cells, white blood cells, platelets, and a wide variety of dissolved molecules The liquid portion of blood is blood plasma Some of the more common solutes in blood plasma are Glucose (blood sugar) Amino acids Fibrinogen (protein involved in blood clotting) Hydrogencarbonate ions (as a means of transporting CO2)