N2.1 Hydrogen bonds
advertisement

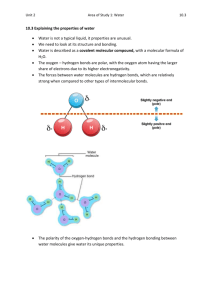
Overview: The Molecule That Supports All of Life • Water is the biological medium on Earth • All living organisms require water more than any other substance • Most cells are surrounded by water, and cells themselves are about 7095% water • The abundance of water is the main reason the Earth is habitable Water is a kinetic energy/ heat energy sponge – Liquid Water can absorb a lot of heat energy without changing temperature. Latent heat of vaporization Latent heat of fusion Water is a kinetic energy/ heat energy sponge – Latent HEAT of FUSION – heat energy that can be released before something will start melting/ becoming a liquid. Note that heat is released but the temperature does not change – Latent Heat of Vaporization – heat energy that can be absorbed before something starts to boil and become a gas. Note that heat is absorbed but the temperature does not change Latent heat of vaporization Latent heat of fusion Heat Capacity of Liquid Water Thermal Properties of Water Physiology: Cooling effects •The heat generated by the human body needs to be removed to prevent a denaturation of enzyme systems. •Water absorbed a great deal of energy before entering the vapour phase. This makes it an effective agent for the removal of heat and the maintenance of body temperature. •Blood which is mainly water can absorb and carry heat away from hot parts of the body to the cooler parts. Ecological effects: •Oceans, lakes and ponds have fairly stable temperature (since it can absorb a lot of heat without a temperature change) which means that poikilothermic organisms need not waste energy on thermoregulation. Surrounding air temperatures may show marked changes but water water in the same vicinity will remain relatively stable Why is water an kinetic/ heat energy spongy? Chemical Properties of water: • Water molecules are strongly ATTRACTED to other water molecules. • This attraction is a type of intermolecular force a force between water molecules called hydrogen bonds. The causes for the Intermolecular force (i.e. Hydrogen Bonds) between water molecules • The water molecule is a polar molecule: The opposite ends have opposite charges • Polarity allows water molecules to form hydrogen bonds with each other _ O H + H + Animation: Water Structure The causes for the Intermolecular force (i.e. Hydrogen Bonds) between water molecules • This image of water show the covalent bonds between oxygen and two hydrogen atoms. • The nuclei of oxygen is significantly larger and greater charge (+8) than the hydrogen nuclei (+1). • Consequently the electron pair in the covalent bond is found 'closer' to the oxygen than the hydrogen nuclei. • This creates a polar molecule in which the oxygen carries and additional small negative dipole and each hydrogen a small positive dipole. Intermolecular force (i.e. Hydrogen Bonds) between water molecules Hydrogen bonds Consequences of Polarity • Due to the polarity of the water molecules, the small negative charge on each oxygen atoms is attracted the hydrogen atoms of other water molecules. • These attractions are called HYDROGEN BONDS. • A hydrogen bond in water has a typical strength of 5.0 kcal/mol but these vary in strength in solutions. • Hydrogen bonds can occur between water molecules and with other types of polar molecule ASSESSMENT STATEMENT • Draw and label a diagram showing the structure of water molecules to show their polarity and hydrogen bond formation. Why is water an kinetic/ heat energy spongy? It takes a lot of energy to break hydrogen bonds. Thus, solid water (ice) and liquid water can absorb a lot of heat energy before they can states and increase in temperature Heating Curve- Thus, solid water (ice) and liquid water can absorb a lot of heat energy before they can states and increase in temperature Four properties of water contribute to Earth’s fitness for life • Four of water’s properties that facilitate an environment for life: 1. Cohesive behavior 2. Ability to moderate temperature 3. Expansion upon freezing 4. Versatility as a solvent Xylem Cells Leaves Cohesion and Adhesion • Water molecules are weakly attracted to each other by hydrogen bonds (Cohesion). This action extends down the xylem creating a 'suction' effect. • There is also adhesion between water molecules and the xylem vessels Xylem Cells Leaves Capillary Action • The cohesion and adhesion act together to maintain the water column all the way up from the root to the stomata • The tendency of water to rise in a thin tube due to adhesion & cohesion is called capillary action Surface Tension is a function of cohesive properties of water • Surface tension is a measure of how hard it is to break the surface of a liquid • Surface tension is related to cohesion Moderation of Temperature Water has four properties that allow it to moderate the temperature of living organisms and the temperature on earth 1. High Specific Heat Capacity 2. High Latent heat of Vaporisation 3. High Latent heat of fusion 4. Ability to Insulate of Bodies of Water by Floating Ice Moderation of Temperature (specific heat capacity) • Liquid water can absorb or release a large amount of heat with only a slight change in its own temperature • The heat energy required to raise a substances temperature is called specific heat capacity. • Liquid Water has a high specific heat capacity. • Thus Liquid water can absorb a lot of heat without changing temperature. Water’s High Specific Heat (specific heat) • Water’s high specific heat minimizes temperature fluctuations to within limits that permit life – Heat is absorbed when hydrogen bonds break – Heat is released when hydrogen bonds form Evaporative Cooling (Latent Heat of vaporization) • Evaporation is transformation of a substance from liquid to gas • Latent Heat of vaporization is the heat a liquid must absorb for 1 gram to be converted to gas • As a liquid evaporates, its remaining surface cools, a process called evaporative cooling • Evaporative cooling of water helps stabilize temperatures in organisms and bodies of water Latent Heat of Fusion • Latent Heat of Fusion -- The amount of heat required to be removed in order for a substance to become a solid. • The latent heat of fusion is very high for water. • Thus when water becomes ice it absorbs an incredible amount of heat. Insulation of Bodies of Water by Floating Ice • Ice floats in liquid water because hydrogen bonds in ice are more “ordered,” making ice less dense • If ice sank, all bodies of water would eventually freeze solid, making life impossible on Earth