Water - 9AcademicScience
advertisement

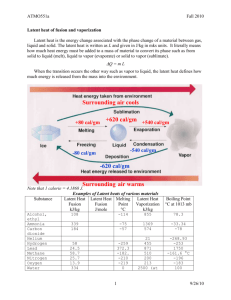
• Water is not an organic molecule but is essential for life on this planet • All cells are surrounded inside and out with water – anything that interacts with a cell must first be dissolved in water • Physical properties: – – – – colourless and transparent liquid at room temperature density = 1.0 g/mL m.p. = 0℃ b.p = 100℃ • water has LD, D-D forces, and H-bonding • Water has cohesive properties – the high number of intermolecular forces causes water molecules to ‘stick’ together Examples: – surface tension – beading of water – water striders – too light to break surface tension – transpiration in plants – transport in xylem tubes • Water has adhesive properties – it’s polar nature causes it to stick to other substances Examples: – capillary action – water ‘climbs’ up small diameter tubes, or ‘bleeds’ through the microscopic pores and channels in paper or other porous substances – this is due to the hydrogen bonding interactions between the water and the surface of the tube (either SiO2 or the cellulose tubes of paper) – This helps to explain the meniscus inside a tube • Water has outstanding solvent properties • Used to be called the ‘universal solvent’, but this is not a good name, since not everything dissolves in water • The polar nature of water allows any other polar substance or any charged particle to dissolve easily • The δ- will attract the δ+ end of solutes, and this attraction will remain once the solute is dissolved. • The same is true for ionic substances – the cation will be attracted to the δ- end of water, and the anion will be attracted to the δ+ of water. • Water has a high specific heat capacity • This is a measure of the amount of heat energy required to increase the temperature of a 1g of a substance by 1℃. • cwater = 4.18 J/g‧℃ • This is high compared to other substances: ccopper = 0.385 J/g‧℃ cglass = 0.735 J/g‧℃ cair = 1.00 J/g‧℃ ciron = 0.450 J/g‧℃ • A metal pan absorbs heat energy quickly and loses it quickly. This makes metals useful for cooking. • Water takes more energy to heat up – thus the time it takes to boil water in a pot. • Moderation of climate • This property of water also helps to moderate temperature changes in cells Special Properties of water • Water has a high latent heat of vaporization and fusion. • Latent heat is the energy absorbed or released by a substance during a change of state. • Lf water = 334 J/g • Lv water = 2260 J/g Latent heat Latent heat of vaporization • Evaporative cooling relies on Lv of water. Latent heat of fusion • Tender fruit farmers take advantage of the latent heat of fusion of ice when there is a chance of frost • On an evening when there is frost in the forecast, they spray water over their fruit, causing ice to form as the temperature drops below 0°C. • How does this help to protect the fruit? Special Properties of water • Water’s density decreases as it changes from liquid to solid. • This is because the distance between molecules in a crystal lattice (as ice) on average further than when in a liquid. HOMEWORK • • • • Read and Summarize Page: 195 “Salt and Ice” Pages: 196-197 Unuaual Properties of Water Questions page 198 # 1,11