Class13
advertisement

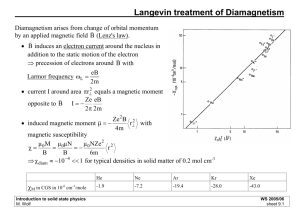
Magnetoresistance and Giant Magnetoresistance and Introduction to Atoms and Energies Magnetic Storage • The smallest region with uniform magnetism is called a “domain” • Each bit requires two domains to allow for error identification • If two domains are magnetized in same direction, the bit is a 0 • If two domains are magnetized in opposite directions, the bit is a 1 • Direction of magnetization must change at the start of each new bit. Magnetic Storage: Writing • Magnetic fields have two sources: – Currents (electromagnetism) – Alignment of intrinsic “spin” of particles (ferromagnetism) • Magnetic data is written by running a current through a loop of wire near the disk – resulting magnetic field aligns spins in region of disk and produces magnetic domain – switching current produces magnetic domain with magnetism in opposite direction Magnetic Storage: Faraday’s Law • A changing magnetic field induces a current in a coil of wire proportional to the derivative (rate of change) of the field with respect to time. • The emf, and current also depend upon the field, area A of the loop, and the number of turns in a coil. • This is summarized in Faraday’s Law: d B iR dt B B dA Magnetic Storage: Reading by Induced Currents • As magnetic data passes by coil of wire, changing field induces currents – increase in field (more positive or less negative) induces current in opposite direction of that induced by a decrease in field (more negative or less positive) – Number of changes in a bit indicates whether bit is 0 or 1 Magnetic Storage: Reading by Magnetoresistance • Charges traveling through magnetic field experience magnetic force (provided velocity and field are not aligned): FB = qv x B • Force is perpendicular to velocity (and to field), so charges are pushed “off track”, resulting in more frequent collisions and thus an increased resistance • Current through a loop of wire near magnetic data will vary as magnetic field does, giving a very sensitive indication of magnetic data Magnetic Storage: Reading by Giant Magnetoresistance • Giant Magnetoresistance (GMR) is a completely different effect from Magnetoresistance (MR) – Both utilize magnetic data’s effect on resistance, but that’s the only similarity • MR is the regular “Lorentz” force on charges moving in a magnetic field • GMR exploits spin-dependent scattering and requires very carefully-crafted devices such as spin valves Spins and ferromagnetism • Ferromagnetism due to spins of electrons • Can classify electrons as “spin-up” or “spindown”, based on the component of magnetic field along a chosen axis Chosen axis (z) Electrons with intrinsic magnetic field indicated Up Down Up Down Up Up Down Spins and Scattering • An electron moving into a magnetized region will exhibit spin-dependent scattering • Electrons with spins in the direction of the magnetic field will scatter less than electrons with spins opposite the direction of the magnetic field Magnetization Magnetic Superlattices • Alternate layers of ferromagnetic material will naturally align with opposite magnetization • All electrons coming in will scatter since they’ll have opposite spin from magnetization in some region Ferromagnetic material with magnetization in direction of turquoise arrow Non-ferromagnetic material spacer Warning: Figure not to Scale Magnetic Superlattice in Field • If an external field is present, ferromagnetic layers will all align with external field • Only half of the electrons coming in will scatter maximally, those with spin opposite external field Externally applied magnetic field Giant magnetoresistance • When magnetic field is present in magnetic superlattice, scattering of electrons is cut dramatically, greatly decreasing resistance • Superlattices are hard to mass-produce, but the effect has been seen in three-layer devices called “spin valves” • The origin of giant magnetoresistance is very different from that of regular magnetoresistance! The Future is Now • Magnetoresistance read heads have been produced at IBM since 1992 • Magnetoresistance read heads have been exclusively used at IBM since 1994 • Giant magnetoresistance spin valves have been used to pack 16.8 gigabytes onto a PC hard drive in 1998 • Currently a density of 35.3 Gbits/in2 has been achieved • IBM is working toward density of 100 Gbits/in2 Stuff to remember about GMR • Electrons (and other elementary “particles”) have intrinsic magnetic fields, identified by spin • The scattering of electrons in a ferromagnetic material depends on the spin of the electrons • Layers of ferromagnetic material with alternating directions of magnetization exhibit maximum resistance • In presence of magnetic field, all layers align and resistance is minimized On To Atoms • Around the turn of the century, Bohr proposed that electrons in atoms can only occupy certain, quantized energy “states” • When an electron moves from one allowed state to another, it needs to absorb or emit a particular amount of energy – Often that energy takes the form of light – Only specific energies (and therefore wavelengths) of light will be emitted by a particular element – The collection of energies emitted or absorbed by an element is called the atomic spectrum of that element Our Model of the Atom • If the atom is in the “ground state” of lowest energy, electrons fill the states in the lowest available energy levels. The first shell has two possible states, and the second shell has eight possible states. Higher shells have more states, but we’ll represent them with the eight states in the first two sub-shells. • Electrons in the outermost shell are called “valence” electrons. We’ll make them green to distinguish from e- in filled shells E=0 (unbound) n=4 n=3 n=2 n=1 Really eight closely spaced energies, since no two electrons can occupy same state The Hydrogen Atom • Has one electron, normally in the ground state n=1 • This electron can absorb energy and go to a higher state, like n=3 • The atom will eventually return to its ground state, and the electron will emit the extra energy in the form of light. • This light will have energy E = (13.6 ev)(1/1 – 1/32) = 12.1 eV • The corresponding wavelength is l = hc/E = 1020 Å E=0 (unbound) n=4 n=3 n=2 n=1 Other Atoms • Electrons can absorb energy and move to a higher level – White light (all colors combined) passing through a gas will come out missing certain wavelengths (absorption spectrum) • Electrons can emit light and move to a lower level • Calculating the allowed energies extremely complicated for anything with more than one electron • But can deduce allowed energies from light that is emitted n=4 n=3 n=2 n=1 E=0 (unbound) Really eight closely spaced energies, since no two electrons can occupy same state Atomic Bonding • Electrons in an unfilled valence shell are loosely bound • Atoms will form bonds to fill valence shells, either by sharing valence electrons, borrowing them, or loaning them • When atoms bond in solids, sharing electrons, each atom’s energy levels get slightly shifted E=0 (unbound) n=4 n=3 n=2 n=1 Before the next class, . . . • Finish Homework 14 • Do Activity 13 Evaluation by Midnight tonight • Read Chapters 2-3 in Turton • Do Reading Quiz Do Today’s Activity What Have We Learned About Atoms? • ENERGY IS QUANTIZED • Electrons can absorb energy and move to a higher level; they can emit light and move to a lower level • In hydrogen the emitted light will have energy E = (13.6 ev)(1/nf2 – 1/ ni2) • The wavelength is given by l = hc/E = 1240(nm eV)/E • Energy levels of nearby atoms are slightly shifted from each other, producing bands of allowed energies • Electrons move from the locality of one atom to the next only if an energy state is available within the same band What Have We Learned About Spectra? • ENERGY IS QUANTIZED • Different elements have different allowed energies (since different numbers of protons and electrons provide different structure of attraction) • Light emitted when electrons move from a high energy level to a lower energy level in an atom will have only certain, QUANTIZED, allowed energies and wavelengths. • Those wavelengths depend solely on the element emitting the light and compose the characteristic emission spectrum for that element