LDL-C and CV Risk - jackrabbitdesign.com
advertisement

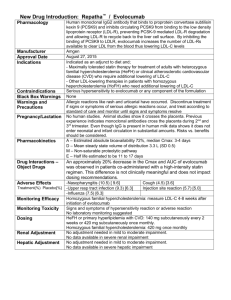
LDL-C and CV Risk: What We Know and Don't Know Joseph J. Saseen, PharmD, FCCP, BCPS, CLS Associate Professor Clinical Pharmacy and Family Medicine University of Colorado Denver 1 American Heart Association News 1/22/2008 Since 1999, death rates have dropped: – Coronary heart disease 25.8% – Stroke 24.4% Drops are ahead of goals set for the year 2010 “However, potential problems loom for the future, as all of the major risk factors for these leading causes of death are still too high and several are actually on the rise.” http://www.americanheart.org/ 2 NHANES: Serum Lipids and Lipoproteins in Adults Mean Value (mg/dL) 225 1976-1980 215 204 203 200 1988-1994 175 1999-2002 150 138 125 129 123 114 118 123 100 75 49.7 50.7 51.3 50 25 0 Total Cholesterol Carroll MD et al. JAMA. 2005;294:1773-1781. HDLCholesterol LDLCholesterol Triglycerides 3 LDL-C and CV Risk 3.7 2.9 30 mg/dL Relative Risk for 2.2 Coronary Heart 1.7 Disease (log scale) 30 mg/dL 30 mg/dL 30 mg/dL 1.3 1.0 40 70 100 130 160 190 LDL-Cholesterol (mg/dL) Grundy SM et al. Circulation. 2004; 110:227-239. 4 Cholesterol Treatment Trialists’ Collaborators Meta-analysis,14 randomized controlled trials (n=90,056) Primary Prevention (no CHD) Major Vascular Events (per 1 mmol/L LDL-C reduction) Control Statin 10.6 Seconday Prevention (Post-MI) 8.5 26.9 21.2 0 5 10 Both comparisons, P<0.001 Baigent C et al. Lancet. 2005;366:1267-1278. 15 20 25 30 Event Rate (% ) 5 Lipid-Lowering Therapies Statins (atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, simvastatin) Bile Acid Sequestrants (colesevelam, cholestyramine, colestipol) Nicotinic Acid Fibric Acid Derivatives LDL-C HDL-C TG 18-63% 5-15% 7-30% 15-30% 3-5% 0 or 5-25% 15-35% 20-50% 5-20 or 10-20% 20-50% (gemfibrozil, fenofibrate) Cholesterol Absorption Inhibitor (ezetimibe) Omega-3 fatty acids 18% 1% 7% ? 9% 45% (prescription strength only) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486-2497. Zetia [package insert]. Merck/Schering-Plough Pharmaceuticals; 2005. Crestor [package insert]. Astra-Zeneca; 2005. Omacor [package insert]. Reliant Pharmaceuticals; 2005. 6 Mechanism of Action – HMG CoA Reductase Inhibitors Acetyl CoA HMG-CoA Competitive Inhibition Mevalonate Cholesterol production Expression of LDL receptors LDL, VLDL, and IDL particles HMG CoA Reductase Inhibitors (Statins) Cholesterol production LDL Lowering 7 STELLAR Trial Statin Therapies for Elevated Lipid Levels Compared Across Doses to Rosuvastatin 6-week, parallel groups, open-label study (n=2431) % LDL-C Change from Baseline Pravastatin Simvastatin Atorvastatin Rosuvastatin 0 -10 -20 -30 -40 -20.1 -24.4 -29.7 10mg -28.3 20mg -35.0 -38.8 -36.8 40mg 80mg -42.6 -50 -45.8 -45.8 -47.8 -51.1 -60 Jones PH et al. Am J Cardiol. 2003;92:152-160. -52.4 -55.0 8 Landmark Statin-based Outcome Trials Trial LDL-C (mg/dL) Primary Endpoint/ CV Event Rate (%) Baseline Treatment Placebo Statin 4S 188 122 28.0 19.4 LIPID 150 112 15.7 12.3 CARE 139 98 13.2 10.2 Primary & Secondary Prevention HPS 132 93 24.4 19.9 PROSPER 147 97 16.2 14.1 Primary Prevention WOSCOPS 192 159 7.5 5.3 AFCAPS 150 115 5.5 3.5 ASCOT 133 90 3.0 1.9 CARDS 118 77 9.0 5.8 Secondary Prevention Jacobson TA et al. Arch Intern Med. 1998;158:1977-1989.; Heart Protection Study Collaborative. Lancet. 2002;360:7-22.; Shepherd J et al. Lancet. 2002; 360:1623-1630.; Sever PS et al. Lancet. 2003;361:1149-58.; Colhoun HM et al. Lancet. 2004;364:685-696. Patients with CHD: Intensive Vs Moderate Statin Therapy Meta-analysis of 4 major trials (PROVE-IT, A to Z, TNT, IDEAL); included 27,548 patients P<0.0001 P<0.0001 P=0.054 Cannon CP et al. J Am Coll Cardiol. 2006;48:438-445. 10 Pleiotropic Effects of Statins? Beneficial CV effects that are not related to LDL-C lowering – Anti-inflammatory effects – Immunomodulatory effects – Endothelial dysfunction improvement Increased nitric oxide bioavailability Decreased LDL-C oxidation – Plaque stability – Inhibiting the thrombogenic response Liao JK, Laufs U. Ann Rev Pharmacol Toxicol. 2005;45:89-118. Tandon V. Indian J Pharmacol. 2005;37:77-85. 11 Mechanism of Action – Bile Acid Sequestrants Bile Acid Sequestrants Hepatic Bile Acid Pool Hepatic Bile Acid Synthesis from Cholesterol Intrahepatic Cholesterol Pool HMG-CoA Reductase Expression LDL Receptors VLDL Production / Secretion LDL Production LDL Clearance Plasma LDL-C 12 Bile Acid Sequestrants (colestipol, colesevelam, cholestyramine) Provide modest reductions in LDL-C May increase triglyceride values, especially in patients with baseline hypertriglyceridemia Avoid systemic toxicities Some can bind the absorption of other drugs when administered simultaneously Primary roles are in addition to statin-based therapy or in statin-resistant patients 13 Bile Acid Sequestrant Outcomes Data LRC-Primary Prevention Trial (n=3086): – Cholestyramine reduced fatal CHD + non-fatal MI 19% versus placebo over 7.4 yrs (7.0 vs 8.6%, P<0.05) FATS Trial (n=146): – Intensive LDL-C lowering in CHD patients using colestipol with lovastatin or niacin lowered CV event risk versus conventional therapy (HR=0.27, 0.10 to 0.77) LRC-CPP Trial. JAMA. 1984;251:351-374. Brown G et al. N Engl J Med. 1990; 323:1289-1298. 14 Mechanism of Action – Fibric Acid Derivatives Liver Fibric Acids (Fibrates) VLDL ApoB HDL LDL TG ApoB 15 Fibric Acid Derivatives (fenofibrate, gemfibrozil) Provide significant reductions in triglycerides and can raise HDL-C Have a limited ability to LDL-C and may paradoxically increase LDL-C Primary roles are for hypertriglyceridemia or in addition to statin-based therapy for mixed dyslipidemia/non-HDL-C reduction CV events reduced in certain primary (Helsinki Heart Study) and secondary prevention populations Frick MH et al. N Engl J Med. 1987;317:1237-1245. 16 2531 men with CHD randomized to placebo or gemfibrozil 1200 mg/day x 5.1 yrs Lipid differences placebo vs gemfibrozil: – HDL: 32 vs 34 – LDL: 113 vs 113 – TG: 166 vs 115 Rubins HB et al. N Engl J Med. 1999;341;410-418. Cumulative incidence (%) Veterans Affairs HDL Intervention Trial (VA-HIT) Death From CHD and Nonfatal MI 25 22% 20 Placebo 15 10 Gemfibrozil 5 0 0 1 2 3 Year 4 5 6 17 Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) 9795 patients with type 2 diabetes – CHD death +nonfatal MI % Patients Randomized, double-blind to placebo or fenofibrate 200mg daily x 5 yrs Primary endpoint: 15 Fenofibrate Placebo P=0.35 10 P=0.16 5 Statin “drop-in” rate was high 0 Primary Endpoint Secondary Endpoint* *Total CV events Keech A et al. Lancet. 2005;366:1849–1861. 18 Mechanism of Action - Niacin Niacin Adipose tissue FA mobilization FA synthesis/ esterification HDL-catabolism receptor TG Synthesis Large TG- rich VLDL Small dense LDL-C Apo B lipoproteins HDL Apo A-I Apo B degradation uptake/removal VLDL, LDL-C HDL Adapted from Kamanna VS, Kashyap ML. Curr Atheroscler Rep. 2000;2:36-46. 19 Nicotinic Acid [a.k.a. Niacin] Changes all lipid components favorably – Consistent LDL-C and triglyceride lowering effects – Raises HDL-C better than any other agent Flushing is minimized with extended-release formulations and other modalities Primary roles are for hypertriglyceridemia or in addition to statin-based therapy for mixed dyslipidemia/non-HDL-C reduction 20 Nicotinic Acid Outcomes Data HATS Trial (n=160) – Simvastatin + niacin reduced CV events versus placebo over 3 yrs in patients (2.6 vs 23.7%, P<0.05) Coronary Drug Project (n=1119) – After 6 yrs, IR niacin (up to 3 g/day) significantly reduced MI compared with placebo in men with CHD – 15 year follow-up data demonstrated reduced mortality ARBITER-2 (n=167) – Significant reductions in carotid IMT with ER Niacin (1 g/day) added to statin therapy versus placebo in patients with CHD Brown BG et al. N Engl J Med. 2001;345:1583-1592.; JAMA .1975;231:360-381.; Canner PL et al. J Am Coll Cardiol. 1986;8:1245-1255.; Taylor AJ et al. Circulation. 2004;110:3512-3517. 21 Mechanism of Action – Cholesterol Absorption Inhibitor 22 Cholesterol Absorption Inhibitor (Ezetimibe) Provides modest reduction in LDL-C Primary role is in addition to statin-based therapy or in statin-resistant patients No definitive outcomes data; however, recent ENHANCE trial has had controversy – 720 patients with heterozygous familial hypercholesterolemia randomized to ezetimibe/simvastatin 10/80 mg daily or simvastatin 80 mg daily for 2 yrs www.theheart.org. 23 ENHANCE: Results Significant differences in LDL-C reduction: – Baseline LDL-C values: – LDL- C reductions: 319 and 318 mg/dL 58 and 41% (P<0.01) Primary Endpoint: Change in mean carotid IMT – Ezetimibe/Simvastatin – Simvastatin 0.0111 mm 0.0058 mm (P=0.29) Other Endpoints: Patients with CV Events – Ezetimibe/Simvastatin – Simvastatin www.theheart.org. 12 of 357 9 of 363 (P=ns) 24 Trial on the Horizon IMPROVE-IT: Examining Outcomes in Subjects With Acute Coronary Syndrome Randomized, double-blind trial comparing ezetimibe/simvastatin 10/40 mg daily vs simvastatin 40 mg daily >10,000 patients who are stable after acute coronary syndrome Primary endpoint: fatal and non-fatal CV event Results expected in 2011 www.clinicaltrials.gov. 25