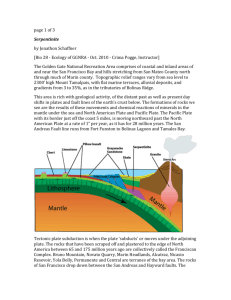
29Serpentine Group
advertisement

Serpentine Group Presentation prepared by Oliver Boyd Serpentine: A Phyllosilicate Formula: Mg3Si2O5(OH)4 End Members: Lizardite, Chrysotile, and Antigorite Serpentine: A Phyllosilicate Serpentine: Structure Member Lizardite Chrysotile Antigorite Crystal System Triclinic T – P31m H1 – P63cm Monoclinic A2/m Monoclinic Pm or P2/m a 5.308 5.313 43.53 b 9.2 9.12 9.26 c 42.712 14.637 7.26 90.0 90.0 90.0 90.0 93.167 91.6 90.0 90.0 90.0 Z 12 4 16 Volume (Å3) 2085.78 708.15 2926.12 Density (kg/m3) 2658 2609 2526 Serpentine: Structure Serpentine: Occurrence Serpentine minerals commonly occur as alterations of magnesium silicates, especially olivine, pyroxene, and amphibole. The following is a possible reaction: Mg2SiO4 + 3H2O Mg3Si2O5(OH)4 + Mg(OH)2 Associated with magnesite, chromite, and magnetite, it is found in both igneous and metamorphic rocks and may make up the entire rock mass. Chrysotile is the asbestiform variety of serpentine. It is extensively mined and is the primary asbestos mineral. Serpentine: Occurrence Fig. 4 from Angel et al. 2001. The maximum thermal stability of hydrous phases in sediment (Ono, 1998), MORB basalt (Schmidt and Poli, 1999), and ultramafic harzburgite (Frost, 1999) bulk compositions. Positions of the –-, –and -Pv + MW transformations for (Mg1.8Fe0.2)SiO4 are shown in gray (Ito and Takahashi, 1989; Katsura and Ito, 1989). An average mantle adiabat (ama), an estimate of the minimum temperature in a subducting slab (cs), and the antigorite breakdown curve (ant) are also shown (Jeanloz and Morris, 1986; Ulmer and Trommsdorff, 1995; Irifune et al., 1996; Stein and Stein, 1996).