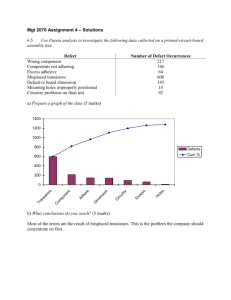
Basic Principles of GMP - World Health Organization
advertisement

QUALITY BY DESIGN Training Workshop on Pharmaceutical Development with focus on Paediatric Formulations Mumbai, India Date: May 2008 Slide 1 May 2008 QUALITY BY DESIGN Dr Tom Sam President Industrial Pharmacy Section International Pharmaceutical Federation (FIP) Slide 2 May 2008 QUALITY BY DESIGN products processes Slide 3 May 2008 What is Quality? Quality Requirements = need or expectations Target Product Quality Profile Patient (or surrogate) “Good pharmaceutical quality represents an acceptably low risk of failing to achieve the desired clinical attributes.” Slide 4 May 2008 Which quality do we want for our medicines ? 6σ DPMO 1000000 Restaurant bills (defectsper 100000 million opportunities) 10000 Airlines baggagecheck in 1000 100 Egypt Air (5,8) Air India(5,8) Lufthansa (6,6) 10 1 0,1 Best-in-class Quantas, SAS 0,01 2σ 3σ 4σ 5σ 6σ 7σ Source: Motorola, Air Safety Online Quelle: Motorola, Air SafetyOnline Slide 5 May 2008 With which quality do we manufacture our medicines: 6σ, 5σ, 4σ, 3σ, 2σ ? DPMO 1000000 Restaurant bills (defectsper 100000 million opportunities) 10000 Airlines baggagecheck in 1000 100 Egypt Air (5,8) Air India(5,8) Lufthansa (6,6) 10 1 0,1 Best-in-class Quantas, SAS 0,01 2σ 3σ 4σ 5σ 6σ 7σ Source: Motorola, Air Safety Online Quelle: Motorola, Air SafetyOnline Slide 6 May 2008 How do we fill this quality gap in the pharmaceutical industry? Sigma ppm Defects Current Mfg Quality provided to patients 2 3 4 5 6 308,537 66,807 6,210 233 3.4 Yield 69.2% 93.3% 99.4% 99.98% 99.99966% Cost of Quality 25-35% 20-25% 12-18% 4-8% 1-3% ……by testing !!!! Data from: Dr. Doug Dean & Frances Bruttin PriceWaterhouseCoopers Slide 7 May 2008 The quality mantra “Quality can not be tested into products; it has to be built in by design” Slide 8 May 2008 How can we modernize our industry? More knowledge of our products and processes, allowing better design and more control Better management: - introduction of quality risk management - expansion of GMP to more extensive pharmaceutical quality system Slide 9 May 2008 Dr Ajaz Hussain ‘Pharmaceutical GMPs for the 21st Century’ Slide 10 May 2008 Slide 11 May 2008 Knowledge based decisions Need for regulatory oversight The knowledge pyramid First Principles Why? MECHANISTIC KNOWLEDGE How? Desired State “CAUSAL" KNOWLEDGE What “Causes” What? CORRELATIVE KNOWLEDGE What Is Correlated to What? DESCRIPTIVE KNOWLEDGE: What? Current State The New Quality Paradigm – The Evolving Regulatory Framework Product Life Cycle Product Design Process Design Scale-up & Transfer Commercial Manufacture ICH Q8/Q8(R) - Pharmaceutical Development PAT Guidance ICH Q9 – Quality Risk Management ICH Q10 – Pharmaceutical Quality Systems Slide 12 May 2008 Product Slide 13 May 2008 Definition: Quality by Design Quality by Design is a systematic approach to development that begins with predefined objectives and emphasizes - product and process understanding - and process control, based on sound science and quality risk management. Slide 14 May 2008 EMEA/CHMP/ICH/518819/2007 Quality by Design approach can be used for Active pharmaceutical ingredients Materials incl excipients Analytics Slide 15 May 2008 Simple dosage forms Advanced drug delivery systems Devices Combination products (e.g. theranostics) Impact of QbD Companies re-organize their science Universities change their curriculum Health authorities change their assessment and inspection Slide 16 May 2008 QUALITY BY DESIGN Step 1. Agree on the Target Product Profile Step 2. Determine the Critical Quality Attributes (CQAs) Step 3. Link the drug and excipient attributes and the process parameters to the CQAs Step 5. Define the Control Strategy Step 6. Prepare QbD registration file Step 7. Product lifecycle management and continual improvement Slide 17 May 2008 EMEA/CHMP/ICH/518819/2007 Step 4. Define the Design Space What are the steps in a Quality by Design approach? 2. CRITICAL QUALITY ATTRIBUTES 3. LINK MAs AND PPs TO CQAS 1. TARGET PRODUCT PROFILE 4. ESTABLISH DESIGN SPACE 6. PRODUCT LIFECYCLE MNGMNT Slide 18 May 2008 5. ESTABLISH CONTROL STRATEGY Step 1. Agree on the Target Product Profile Target Product Profile: - a prospective and dynamic summary of the quality characteristics of a drug product - that ideally will be achieved to ensure that the desired quality, and hence the safety and efficacy, of a drug product is realised. The TPP forms the basis of design of the product. Slide 19 May 2008 Consider: dosage form route of administration strength release / delivery of the drug pharmacokinetic characteristics (e.g., dissolution; aerodynamic performance) drug product quality criteria (e.g., sterility, purity). TPP for paediatric dosage form TPP adult Slide 20 May 2008 TPP paediatric (may depend upon age group) What are the steps in a Quality by Design approach? 2. CRITICAL QUALITY ATTRIBUTES 3. LINK MAs AND PPs TO CQAS 1. TARGET PRODUCT PROFILE 4. ESTABLISH DESIGN SPACE 6. PRODUCT LIFECYCLE MNGMNT Slide 21 May 2008 5. ESTABLISH CONTROL STRATEGY CRITICAL QUALITY ATTRIBUTES - definition A critical quality attribute (CQA) is a - physical, chemical, biological, or microbiological property or characteristic - that should be within an appropriate limit, range, or distribution - to ensure the desired product quality. EMEA/CHMP/ICH/518819/2007 Slide 22 May 2008 Step 2. Determine the Critical Quality Attributes (CQAs) Drug product CQAs are used to guide the product and process development. solid oral dosage forms: typically those aspects affecting - product purity - product potency - product stability - drug release. Slide 23 May 2008 other delivery systems: can additionally include more product specific aspects, such as - aerodynamic properties for inhaled products - sterility for parenterals, - adhesive force for transdermal patches. Product-centric Quality by Design Excipient Quality Attributes DRUG PRODUCT Formulation Parameters Chemical purity Physical form API Purity Raw Material quality Formulation Process Related Particle size API Quality Attributes Mechanical Properties Excipient Compatibility Slide 24 May 2008 What are the steps in a Quality by Design approach? 2. CRITICAL QUALITY ATTRIBUTES 3. LINK MAs AND PPs TO CQAS 1. TARGET PRODUCT PROFILE 4. ESTABLISH DESIGN SPACE 6. PRODUCT LIFECYCLE MNGMNT Slide 25 May 2008 5. ESTABLISH CONTROL STRATEGY Step 3. Link the drug and excipient attributes and the process parameters to the CQAs I Chart 115 UCL=111.55 Individual Value 110 105 _ X=99.63 100 95 90 LCL=87.71 60 62 64 66 68 70 72 Observation 74 76 78 80 People I Chart 115 UCL=112.65 Inputs to the process control variability of the Output 110 Individual Value 105 100 _ X=97.94 95 90 85 LCL=83.23 80 40 44 46 48 50 52 Observation 54 56 58 60 Equipment I Chart 115 UCL=112.65 110 Individual Value 105 100 _ X=97.94 95 90 85 LCL=83.23 y = ƒ(x) I Chart 80 40 42 44 46 48 50 52 Observation 54 56 58 60 115 Measurement UCL=116.68 Individual Value 115 110 105 _ X=102.37 100 95 UCL=114.17 110 y Individual Value I Chart 120 105 _ X=99.95 100 95 90 LCL=88.05 20 22 24 26 28 30 32 Observation 34 36 38 40 90 Process LCL=85.72 85 1 I Chart 115 11 21 31 41 51 61 Observation 71 81 91 UCL=111.55 110 Individual Value I N P U T S (X) 42 105 _ X=99.63 100 95 90 OUTPUT LCL=87.71 60 62 64 66 68 70 72 Observation 74 76 78 80 Materials I Chart UCL=111.17 110 Individual Value 105 _ X=98.76 100 95 90 LCL=86.35 85 80 82 84 86 88 90 92 Observation 94 96 98 100 Environment Slide 26 May 2008 Source: Moheb Nasr, FDA Mapping the Linkage Inputs: Outputs: CQA1 M2 Material Attributes Critical CQA2 Quality Attributes CQA3 P1 P2 P3 Process Parameters Relationships: CQA1 = function (M1) CQA2 = function (P1, P3) CQA3 = function (M1, M2, P1) P2 might not be needed in the establishment of design space Slide 27 May 2008 Source: Moheb Nasr, FDA M1 Experimental Approach for Identifying Parameters Design of Experiments (DOE) is an efficient method to determine relevant parameters and interactions 2. Conduct randomized experiments 1. Choose experimental design (e.g., full factorial, d-optimal) 3. Analyze Data Determine significant factors Slide 28 May 2008 Experiment Factor A Factor B Factor C 1 + - - 2 - + - 3 + + + 4 + - + ICH Q9 Quality Risk Management Initiate Quality Risk Management Process Formal Risk Management Process 1. Risk 2. Risk Assessment Control Output / Result of the Quality Risk Management Process 4. Slide 29 May 2008 Risk Review What are the steps in a Quality by Design approach? 2. CRITICAL QUALITY ATTRIBUTES 3. LINK MAs AND PPs TO CQAS 1. TARGET PRODUCT PROFILE 4. ESTABLISH DESIGN SPACE 6. PRODUCT LIFECYCLE MNGMNT Slide 30 May 2008 5. ESTABLISH CONTROL STRATEGY Step 4. Define the Design Space The linkage between - the process inputs (input variables and process parameters) and - the critical quality attributes can be described in the design space. Slide 31 May 2008 Definition of Design Space The multidimensional combination and interaction of input variables (e.g. material attributes) and process parameters that have been demonstrated to provide assurance of quality. Roll pressure The material attributes and process parameters that assure quality. Gap width Screen Size Dataset - Run1-10a.M3 Observed vs. Predicted $Time [Last comp.] (Aligned) 300 200 100 0 -100 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 250 260 $Time (normalized) SIMCA-P+ 11.5 - 05/02/2007 23:17:07 Slide 32 May 2008 What are the steps in a Quality by Design approach? 2. CRITICAL QUALITY ATTRIBUTES 3. LINK MAs AND PPs TO CQAS 1. TARGET PRODUCT PROFILE 4. ESTABLISH DESIGN SPACE 6. PRODUCT LIFECYCLE MNGMNT Slide 33 May 2008 5. ESTABLISH CONTROL STRATEGY 1a Response surface plot of in-vitro release as a function of two critical parameters of the mixing and lamination process. Slide 34 May 2008 Contour plot of in-vitro release EMEA/CHMP/ICH/518819/2007 Design Space Design Space Design Space Knowledge Space Control Space Slide 35 May 2008 Step 5. Define the Control Strategy The control strategy should describe and justify how in-process controls and the controls of - input materials (drug substance and excipients), - container closure system, - intermediates and the controls of end products contribute to the final product quality Slide 36 May 2008 5. CONTROL STRATEGY Elements of a control strategy can include, but are not limited to, the following: • Control of input material attributes (e.g., drug substance, adhesive polymer, primary packaging materials) based on an understanding of their impact on processability or product quality • Product specification(s) • Controls for unit operations that have an impact on downstream processing or end-product quality (e.g., the impact of solvent on degradation) • In-process or real-time release in lieu of end-product testing • A monitoring program (e.g., full product testing at regular intervals) for verifying multivariate prediction models. Slide 37 May 2008 What are the steps in a Quality by Design approach? 2. CRITICAL QUALITY ATTRIBUTES 3. LINK MAs AND PPs TO CQAS 1. TARGET PRODUCT PROFILE 4. ESTABLISH DESIGN SPACE 6. PRODUCT LIFECYCLE MNGMNT Slide 38 May 2008 5. ESTABLISH CONTROL STRATEGY Step 7. Product lifecycle management continual improvement Minimal Approach • Reactive (i.e., problem solving and corrective action) Slide 39 May 2008 QbD Approach • Preventive action • Continual improvement facilitated Better processes will lead to products with less variability Now (GMP) Variable Input Fixed Process Variable Output Drug Product PAT/QbD Variable Input Slide 40 May 2008 Adapted Process Consistent Output The Revolution in Quality Thinking Quality by Testing and Inspection Enhanced • product knowledge • process understanding Quality by Design quality assured by well designed product & process Slide 41 May 2008