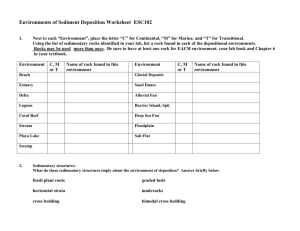
Earth Systems 3209
Unit: 3
Earth’s Materials
Reference:
Chapters 2, 3, 6, 7; Appendix A & B
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Unit 3:
Topic 4.5
Chemical Sedimentary Rocks
Focus on . . .
identifying the processes that form chemical
sedimentary rocks.
contrasting precipitates vs. evaporites.
identifying different chemical sedimentary rocks and the
environment in which they form.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
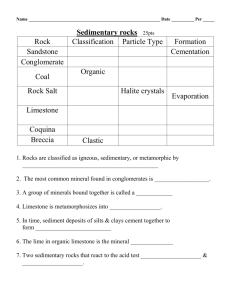
Chemical Sedimentary Rocks
These rocks form as a result of chemical weathering dissolving
chemicals and transporting it in solution. When conditions are
right, these dissolved chemicals change back into a solid through
the processes of:
1) Precipitation
2)
Evaporation
Evaporation and Precipitation often work together. As water
evaporates, chemicals in solution will precipitate.
Example: Rock Salt (Halite).
These rocks can also form where ground water dissolves
chemicals and precipitates the material in certain environments.
An example of this process would be the formation of
stalactites and stalagmites.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
ChemicalSedimentary Rocks (Precipitation)
Precipitates
Process where a change in environmental
conditions cause chemicals dissolved in solution, to
fall out of solution wich forms a solid material.
Results from a change in environmental conditions,
such as:
• Temperature
• Concentration
• Chemical changes
Most common in shallow water environments.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Chemical Sedimentary Rocks (Precipitation)
Examples of chemical sedimentary rocks include:
1) Limestone
10% of all sedimentary rocks (by volume)
Most abundant chemical sedimentary rock
Composed primarily of calcite (calcium carbonate CaCO3)
Formed by marine organisms (corals, clams, algae)
Some deposited directly out of ocean or other waters
Most abundant chemical precipitate rock which forms in
shallow marine waters. Often contain shell fossils.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Chemical Sedimentary Rocks (Precipitation)
Examples of chemical sedimentary rocks include:
2) Dolomite
CaMg(CO3)2, Calcium Magnesium Carbonate.
Dolomite appears to form in many different
types of environments.
Dolomite is used as an ornamental stone and a
concrete aggregate.
It is an important Petroleum reservoir rock.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Chemical Sedimentary Rocks (Precipitation)
Examples of chemical sedimentary rocks include:
3) Travertine
A kind of limestone deposited by springs.
Groundwater traveling through limestone beds
dissolves calcium carbonate and as this dissolved
matter precipitates it builds up travertine deposits.
"Travertine" is sometimes used to mean cavestone,
the calcium carbonate rock that makes up stalactites
and other cave formations.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Chemical Sedimentary Rocks (Precipitation)
3) Travertine
Stalactite: icicle-like pendants
that hang from the ceilings of
caverns and form where water
seeps through cracks above.
Stalagmite: mound shaped
deposits that form on the floors
of caverns and build upwards
toward the ceilings.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Chemical Sedimentary Rocks (Evaporites)
Evaporites
Process where there is a change in state from
a liquid to a gas, water evaporates.
Chemicals dissolved in the liquid (water) are
left behind as a solid material.
Most common in marine environments, but can
also form in some lakes.
Examples include;
Salt (Halite), Gypsum and Sylvite.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Chemical Sedimentary Rocks (Evaporites)
Examples of chemical sedimentary rocks include:
4)
Rock Salt
Consist of the mineral Halite.
Forms by evaporation of shallow seas and
lagoons that have high concentrations of
halite in solution. The mineral precipitates
out of solution as the water evaporates.
Common use is table salt and road salt.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Chemical Sedimentary Rocks (Evaporites)
Examples of chemical sedimentary rocks include:
5)
Rock Gypsum
Consist of the mineral Gypsum.
Forms by evaporation of shallow seas and
lagoons that have high concentrations of
gypsum in solution. The mineral precipitates
out of solution as the water evaporates.
"Common use is plaster and gyproc (drywall).
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Chemical Sedimentary Environments
Summary
Environment
Rock Types
Shallow Marine
gypsum, halite (rock salt), sylvite,
limestone, dolomite
Deep Marine
(Ocean)
limestone, dolostone, chert
Cave
Travertine
(stalactites and stalagmites)
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Example 1:
Which is an icicle-like, depositional feature common
to cave ceilings?
(A) breccia
(B) stalactite
(C) stalagmite
(D) travertine
Which is a chemical sedimentary rock?
(A) conglomerate
(B) limestone
(C) sandstone
(D) shale
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Example 2:
Under which condition would a chemical sedimentary rock
form?
(A)
(B)
(C)
(D)
cementing of sand and pebbles
compaction of sand and clay
hot chemical fluids changing a minerals composition
precipitation of dissolved materials into solid form
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Your Turn . . .
Take the time and complete the following questions . . .
(Solutions to follow)
Questions:
Using an example, describe the formation of
chemical sedimentary rocks.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Solutions . . .
Questions:
Using an example, describe the formation of chemical sedimentary rocks.
Answer:
Dissolved chemicals in solution (water) can form solid minerals
by precipitating out of solution or as a result of evaporation.
Example:
Salt water has an accumulation of salt dissolved in the oceans.
In shallow marine environments, where water evaporates, the
salt (halite) precipitates and forms rock salt.
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador
Summary . . .
Overview of Points covered:
Chemical Sedimentary
rocks include;
Limestone
Dolomite
Travintine
Rock Salt
Rock Gypsum
Chemical Sedimentary
environments include;
Shallow Marine
Deep Marine (Ocean)
Caves
Copyright © 2014 All rights reserved, Government of Newfoundland and Labrador