Unit Eight: Heat
advertisement

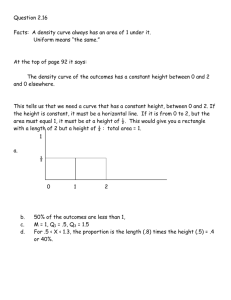
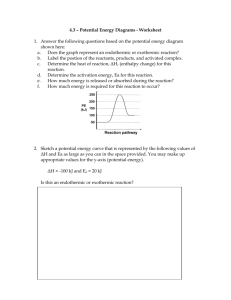
Unit Eight: Heat https://sites.google.com/site/hoyathermochemistry/ What is temperature? • A measure of the average kinetic energy of the particles of a sample; how fast the particles are moving • Common temperature units are Fahrenheit, Celsius, and Kelvin. • We’ll use Celsius. Heat is NOT Temperature. • Heat energy flows from areas of high energy to areas of low energy. • Heat will continue to flow until all areas have reached an EQUAL temperature. Heat is ENERGY that FLOWS. What units measure heat? • • • • • calories Calories (nutritional calories) Joules 4.184 J = 1 cal 1000 cal = 1 Cal The amount of heat that flows can be measured. Exothermic Reactions: • Release heat energy to the surroundings • Cause test tubes to feel hot • Products have less heat than the reactants • Show an overall loss of energy (negative heat change) Endothermic Reactions: • Absorb heat energy from the surroundings • Cause test tubes to feel cold • Products have more heat than the reactants • Show an overall gain of energy (positive heat change) Review: Exothermic & Endothermic Reactions Review: Exothermic & Endothermic Reactions • Look at the reaction described below: 2S + 3O2 --> 2SO3 ∆H = -791.4 kJ • Analyze the reaction: 1. Is heat absorbed or released? 2. What conversion factors could be written to include the heat? • Here is a synonymous reaction: 2S + 3O2 --> 2SO3 + 791.4 kJ Enthalpy values in the product = EXOTHERMIC Enthalpy values in the reactant = ENDOTHERMIC Heat Stoichiometry 2S + 3O2 --> 2SO3 ∆H = -791.4 kJ 2S + 3O2 --> 2SO3 + 791.4 kJ • ∆H can be used as a conversion factor with the coefficients from the equation. • 2 mole S = 791.4 kJ lost • 3 mole O2 = 791.4 kJ lost • 2 mole SO3 = 791.4 kJ lost Understanding the Equation • How much heat will be released when 6.44 g of sulfur reacts with excess O2 according to the equation above? 2S + 3O2 --> 2SO3 ∆H = -791.4 kJ • Since you have a chemical reaction, you have to use stoichiometry. • Label the equation according to the question. • Write the given and draw the chart. • Change grams to moles LIKE ALWAYS. • Use a ratio from the equation to convert moles to energy. Calculations Using ∆H As A Conversion Factor • Solve the 12-2 Practice Problems in your packet. Stoichiometry Practice Heat flow in PHYSICAL CHANGES • Phase changes involve a flow of heat that can be measured. • Solid to liquid to gas • Melting & Evaporation • Gas to liquid to solid • Freezing & Condensation Learning How to Calculate Heat Flow Heating Curve of Water Endothermic graph • • • • • The graph shows heat continually flowing into this sample of water. Solid = heat in causes particles to increase in speed (ΔTemp) Melting = heat in causes intermolecular forces to weaken Liquid = heat in causes particles to increase in speed (ΔTemp) Boiling = heat in causes intermolecular forces to break Gas = heat in causes particles to increase in speed (ΔTemp) Conceptual Understanding Two Equations Used to Calculate Heat Flow q = mcΔT q = molΔH You must be able to recognize a change in temp vs. a change in heat content. Quantitative Understanding • Diagonal Lines: • show heat causing changes in temperature • q = mcΔT • Plateaus: • Show heat causing changes in heat content that weaken/break intermolecular forces without affecting temperature • q = molΔH Putting It All Together • A 5.0 gram sample of water at -40C is heated to 140C. How much heat is required? Let’s use the constants listed on the front of your practice packet. The Calculation • Calculate ONE segment at a time. • Watch the units carefully. • Add the five segments together in the end. • What if the question had said, “A 5.0 gram sample of water at 140C is cooled to -40C. How much heat is released?” • What would the graph look like? • How would the calculation be different for each segment? • Would the graph be endothermic or exothermic? What if…? • What if the question had said, “A 5.0 gram sample of water at 140C is cooled to -40C. How much heat is released?” • What would the graph look like? • The graph would start at 140C and have a negative slope to 40C. • How would the calculation be different for each segment? • The numbers would NOT change at all. However, the changes in temperature and heat content should be negative. • Would the graph be endothermic or exothermic? • The graph shows heat being lost or released out of the sample so it is exothermic. What if…? Answers • A 3.6 gram sample of water is cooled from 75C to -5C. How much heat is released? • Draw the x-axis (time) and y-axis (temp). • Mark the highest and lowest temperatures on the y-axis. • Mark the phase change temperatures that will occur within the temperature range on the y-axis. • Draw the line segments from starting to ending temperature. Be sure to show plateaus at the phase change temps. What if you had to draw your own graph? Temperature 75 A 3.6 gram sample of water is cooled from 75C to -5C. How much heat is released? 0 -5 Time Check your work. • Solve the Heating Curve of Water calculations on the first page of your practice packet. Give each answer to 4 sig figs. • NOTE: The questions will take you step-by-step through the process. Suggested Homework • Question 1: segments. • Question 2: • Question 3: • Question 4: • Question 5: • Question 6: 158.1J • Question 7: We’ll answer as we go through the (3.1g)(2.1J/gC)(0C- -20C) = 130.2J (0.17mol)(6.01kJ/mol) = 1.022kJ (3.1g)(4.18J/gC)(100C-0C)= 1296J (0.17mol)(40.7kJ/mol) = 6.919kJ (3.1g)(1.7J/gC)(130C-100C) = 9525J or 9.525kJ Check your HW: Heating Curve of Water • Goal 1: With a small group, draw a heating or cooling curve to represent a sample. Calculate the heat required to make the temperature/phase changes on the curve. Everyone is responsible for the graph, calculations, and explaining the process. • Goal 2: In a new small group, explain your graph and calculations. Learn about the graphs and calculations for five more samples. • Goal 3: Return to your desk to draw and calculate a curve independently. Select answers in I-Respond. Heating Curve Jig-Saw 1) 2) 3) 4) Assign each group member a number (1-4). Draw the heating or cooling curve to reflect your sample. Calculate the heat flow described in your question and curve. Answer the following questions: a) Is the sample undergoing endothermic or exothermic heat flow? b) Will the q value be positive or negative? c) Will the ΔH value be positive or negative? 5) Discuss the curve and calculations as a group until ALL members are comfortable explaining them to another student. 10 minute time limit Goal 1 Group Instructions 1) Open your Goal 2 folder and pull out the six heating or cooling curve examples. 2) Draw and discuss calculations of Curve 1. (5 minute) 3) Draw and discuss calculations of Curve 2. (5 minute) 4) Draw and discuss calculations of Curve 3. (5 minute) 5) Draw and discuss calculations of Curve 4. (5 minute) 6) Draw and discuss calculations of Curve 5. (5 minute) 30 minute time limit Goal 2 Group Instructions • A sample of 4.9 grams of water is cooled from 114°C to -8°C. Give your answers to four sig figs in Joules. • • • • • ∆H fusion = 6.02 kJ/mol ∆H vap = 40.6 kJ/mol C solid = 2.03 J/g°C C liquid = 4.184 J/g°C C vapor = 1.7 J/g°C Independent Heating Curve Question (10 minute time limit) Is the sample undergoing endothermic or exothermic heat flow? A.) endothermic B.) exothermic Will the q value be positive or negative? A.) positive B.) negative Will the ΔH value be positive or negative? A.) positive B.) negative How much heat is lost in the gas segment of the graph? A.) 949.6 J B.) 287.0 J C.) -139.3 J D.) -116.6 J How much heat is lost in the condensation segment of the graph? A.) -11,040 J B.) 11,040 J C.) 1.640 J D.) -1.640 J How much heat is lost in the liquid segment of the graph? A.) 833.0 J B.) -2050. J C.) -994.7 J D.) 245.3 J How much heat is lost in the freezing segment of the graph? A.) 29.50 J B.) -11.04 J C.) -1,637 J D.) 198.9 J How much heat is lost in the solid segment of the graph? A.) -79.58 J B.) 66.64 J C.) 164.0 J D.) -4.420 J Calculate the total heat required to make the temperature change from 114C to -8C. A.) -3894 J B.) -2259 J C.) -12,250 J D.) -14,920 J • A phase diagram gives the conditions of temperature and pressure at which a substance exists as solid, liquid, and gas. • Each of the three regions represents a pure phase (not a mix). • Each line represents the temp & pressure conditions where the phases exist in equilibrium and phase changes occur. • Triple point: set of conditions in which all phases exist in equilibrium Phase Diagram • If you had a bottle of X in your closet, what state of matter would it be in? • At what temperature and pressure will all three phases exist together? • If I have a bottle of X at 45 atm and 100C, what will happen if I raise the temperature to 400C? • Why can’t the substance be boiled at 200C? Don’t forget to practice the phase diagram questions in your packet! q = m c ΔT Concept Video Specific Heat Capacity q = m c ΔT • c= Amount of heat required to raise 1 g of the substance by 1 degree Celsius. (J/gC) • Specific to a substance; can be used to identify substances as a result • Example 10.4 A 1.6g sample of a metal that has the appearance of gold requires 5.8 J of energy to change its temperature from 23°C to 41°C. Is the metal pure gold? c of Au = 0.129 J/gC. • Specific Heat WS (Practice Packet) 1. A 15.75-g piece of iron absorbs 1086.75 J of heat energy, and its temperature changes from 25°C to 175°C. Calculate the heat capacity of iron. Specific Heat Capacity Water c = 4.184 J/g°C ∆Hfusion = 6.02 kJ/mol ∆Hvaporization = 40.6 kJ/mol • Example 14.1 Calculate the energy required to melt 8.5 g of ice at 0°C. • Example 14.2 Calculate the energy (in kJ) required to heat 25 g of liquid water from 25°C to 100°C and change it to steam at 100°C. • Section Review Question 7 Calculate the energy required to change 1.00 mol of ice at -10°C to water at 15°C. Specific Heat Capacity in Other Calculations • So far, I’ve just told you that heat is added or released from a substance. I haven’t included where the heat is coming from or going. • Example: A 25.0 g sample of pure iron at 85°C is dropped into 75 g of water at 20°C. What is the final temperature of the water-iron mixture? • What direction will heat flow in this example? Fe to H2O or H2O to Fe? • When will the heat stop flowing? • When we know about BOTH parties involved in heat flow, we can calculate many variables. Heat Exchange Between Two Substances • Heat will ALWAYS flow from hot to cold. • Heat will ALWAYS stop flowing when the same final temperature is reached. • If the system is insulated, the amount of heat lost by the hot substance will equal the amount of heat gained by the cold substance. • qlost + qgained = 0 Understanding Heat Flow Between Two Substances • In analytical chemistry labs, a calorimeter is used to insulate heat exchange situations. • We’ll assume that any exchanges calculated in here are insulated in a calorimeter. • Therefore, qlost by the hot substance will equal the qgained by the cold substance. qlost + qgained = 0 Insulated Heat Exchange • A 25.0 g sample of pure iron at 85°C is dropped into 75 g of water at 20°C. What is the final temperature of the water-iron mixture? (cFe = 0.45 J/gC; cH2O = 4.18J/gC) • qlost + qgained = 0 • qFe + qH2O = 0 • (mcΔT)Fe + (mcΔT)H2O = 0 Calculating Heat Flow Between Two Substances Chemistry Thermo WS of Practice Problems 16. The specific heat capacities of Hf and ethanol are 0.146J/gC and 2.45J/gC, respectively. A piece of hot Hf weighing 15.6 g at a temperature of 160.0C is dropped into 125 g of ethanol that has an initial temperature of 20.0C. What is the final temperature that is reached, assuming no heat loss to the surroundings? Another Example • A sample of silver with a mass of 63.3 g is heated to a temperature of 111.4ºC and placed in a container of water at 17ºC. The final temperature of the silver and the water is 19.4°C. Assuming no heat loss, what mass of water was in the container? The specific heat of water is 4.184 J/gºC, and the specific heat of silver is 0.24 J/gºC. • Mass = 139.3 grams A Third Example • An unknown substance at 152C is dropped into H2O at 25C. The mass of the unknown is 12g, and the water has a mass of 100g. If the final temperature of the mixture is 32C, what is the specific heat capacity of the unknown substance? • C = 2.03 J/gC Final Example A rectangular aquarium, 37.4 cm by 30.7 cm by 67.7 cm, is filled with water at 13.5C. How much energy is required to raise the temperature of the water to 22.3C? (1cm3 = 1 mL = 1 gram; cH2O = 4.18J/gC) A.) 1,375 J B.) 324,918 J C.) 2, 859, 278 J D.) Not enough information to calculate How much heat does a 23.0 g ice cube absorb as its temperature increases from -17.4°C to 0.0 °C ? The specific heat of ice is 2.1 J/gC. A.) 840.42 J B.) 1,673 J C.) 84.0 J D.) Not enough information A sample of an unknown metal has a mass of 120.7 g. As the sample cools from 90.5 °C to 25.7 °C , it releases 7020J of energy. What is the specific heat of the sample? A.) -0.8975 J/gC B.) 0.8975 J/gC C.) 1.114 J/gC D.) -1.114 J/gC True or false. Temperature increases as a sample of silver melts. A.) True B.) False Calculate the heat required to vaporize 6.5g of gold. The specific heat capacity of gold is 0.129 J/gC. The ΔHfus is 12.5 kJ/mol, and the ΔHvap is 334.4 kJ/mol. A.) 0.4124 kJ B.) 0.8385 kJ C.) 11.03 kJ D.) 0.0043 kJ What are the temperature and pressure coordinates of the triple point? A.) 50 atm & 350C B.) 90 atm & 750C C.) 40 atm & 400 C D.) 22 atm & -10C • DO NOT SIT DOWN! • DO NOT EAT CHIPS IN THE LAB AREA! • EVAPORATING DISH WILL BE HOT! AFTER THREE TRIALS, • CLEAN UP YOUR STATION AND RETURN TO THE CLASSROOM AREA. • WORK WITH YOUR GROUP TO ANSWER THE LAB QUESTIONS. • ALSO, SOLVE THE “INTERPRETING GRAPHICS” HANDOUT ON THE BACK OF YOUR PRACTICE PACKET. Lab Details • So far, we’ve been analyzing temperature changes and calculating the heat involved in these PHYSICAL changes. • Now, we are going to transition back to chemical changes...chemical reactions. Look at the reaction described below: 2S + 3O2 --> 2SO3 ∆H = -791.4 kJ • Analyze the reaction: 1. Is heat absorbed or released? 2. What conversion factors could be written to include the heat? Heat Stoichiometry 2S + 3O2 --> 2SO3 ∆H = -791.4 kJ 2S + 3O2 --> 2SO3 + 791.4 kJ • ∆H tells if the reaction is endothermic or exothermic. + = endo; - = exo • ∆H can be used as a conversion factor with the coefficients from the equation. • 2 mole S = 791.4 kJ lost • 3 mole O2 = 791.4 kJ lost • 2 mole SO3 = 791.4 kJ lost • ∆H can also be written in the equation. • - = exo = product • + = endo = reactant Understanding the Equation • How much heat will be released when 6.44 g of sulfur reacts with excess O2 according to the equation above? 2S + 3O2 --> 2SO3 ∆H = -791.4 kJ • Since you have a chemical reaction, you have to use stoichiometry. • Label the equation according to the question. • Write the given and draw the chart. • Change grams to moles LIKE ALWAYS. • Use a ratio from the equation to convert moles to energy. Calculations Using ∆H As A Conversion Factor • Solve the 12-2 Practice Problems in your packet. Stoichiometry Practice • Use the specific heat capacity to identify your unknown metal sample. • What equation will you use to find the specific heat capacity? • So, what measurements should you make in the lab to plug into the equation? • You’ll have to use a second substance (water) to calculate a q value to use in your equation. • q lost by the metal + q gained by the water = 0 • A calorimeter is an instrument used to insulate the heat exchange. We’ll build one for your lab. Which is your metal? Specific Heat Capacity Lab • Hot Water Bath to Initially Heat the Metal • Hot plate turned up to 10 • 500mL beaker of water • Test tube containing metal with test tube tongs • Calorimeter to Measure Heat Exchange • Two foam cups stacked • 75mL of water inside • Lid with a hole for thermometer and/or stirring rod • Thermometer to measure initial temp of water Set Up • Follow steps 1-3 to set up your calorimeter. • Replace steps 4-7 with the following: 4. Add 300 mL of water to a 500 mL beaker, and place the beaker on a hot plate. 5. While water is heating, put your sample into a test tube. Use test tube tongs to hold the tube. 6. Place the test tube holding the metal sample in the hot water bath. Allow the metal to remain in the water for three minutes while the water boils. Record the temperature of the boiling water. 7. After three minutes, quickly pour the metal sample from the test tube into the 75 mL of water in the calorimeter. • Follow steps 8-10 to complete the lab. Procedure • Answer the “Analysis and Conclusions” questions. • If the question requires a calculation, please show all of your work. Be organized so that you can receive full credit. Do not leave off units in your answer! • Each group member will turn in his/her own lab report. • Questions will be posted on the blog for those who do not finish in class.