Sugars
advertisement

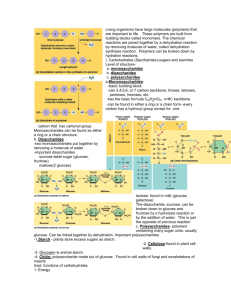
Complex Structures and Functions of Carbohydrates • Monosaccharides- single sugars – Glucose C6H12O6 – Fructose •Disaccharides- double sugars –Sucrose ( table sugar) •Polysaccharides- three or more monosaccharides –Macromolecule –Starch –Glycogen –Cellulose- provides structure for plants (humans cannot digest) Glucose and other monosaccharides often form ring structures #5 #1 The ring forms when a hydrogen is transferred from the hydroxyl group on the #5 carbon to the oxygen of the carbonyl group on the #1 carbon. This allows the #1 carbon to form a bond with the oxygen attached to the #5 carbon, completing the ring. Give an example of a monosaccharide. Give an example of a disaccharide. Give an example of a polysaccharide. What is the molecular formula for this sugar, and how do you know it is a sugar? Two [or more] monosaccharides may be joined by dehydration synthesis to produce larger disaccharides and polysaccharides. Dehydration means to take water out. When dehydration synthesis occurs, something is being built, while taking water out, or producing water as a product. XOH + HZ XZ + HOH Dehydration synthesis is a typical condensation reaction. The reverse reaction of breaking up polymers is accomplished by another chemical reaction known as hydrolysis. Hydrolysis is simply the reverse of dehydration synthesis. You add water to a molecule to break it down. X + H2O --> HX + OH Glucose is a very common monosaccharide that is used for cellular respiration among other things. The names of most sugars end in –ose. The carbon skeletons of monosaccharides are usually 3-7 carbon atoms long. Glucose and fructose are hexoses (six-carbons long), pentoses have five carbons, and trioses have three carbons. Two glucose molecules can be joined to produce a molecule of maltose [and a molecule of water]. A glycosidic link occurs when two monosaccharides are joined by dehydration synthesis. A second disaccharide, the common table sugar known as sucrose, is produced by combining glucose and fructose. If dehydration synthesis continues for a long time, a long and complex carbohydrate chain called a polysaccharide is formed. Polysaccharides are produced by adding more monosaccharides to the chain. Some of the most important polysaccharides are made of long polymers of glucose. What element can be found at each corner in the ring? In glycogen, or animal starch, the glucose units are again joined to produce long chains, but side chains are linked to the main chain. What process joins two monosaccharides together? What type of reaction is dehydration synthesis? When two monosaccharides are joined together, what are the products? What reaction breaks down polysaccharides? What are the reactants in hydrolysis? Where does a glycosidic link form? Storage Polysaccharides Chloroplast Starch • Starch – Is a polymer consisting entirely of glucose monomers – Is the major storage form of glucose in plants 1 m Amylose Amylopectin Plastids are organelles such as chloroplasts, which store starch. Glycogen is the storage form of glucose in animals and humans which is analogous to the starch in plants. Glycogen is synthesized and stored mainly in the liver and the muscles Plants make glucose and cellulose through the photosynthesis processes, and store starch primarily in their roots. Animals in turn eat plant materials and products. Digestion is a form of hydrolysis where the starch is broken down into the various monosaccharides. A major product is glucose, which can be used immediately for metabolism to make energy, through the process of cellular respiration. Mitochondria Giycogen granules 0.5 m Glycogen The glucose that is not used immediately is converted in the liver and muscles into glycogen for storage. Any glucose in excess of the needs for energy and storage as glycogen is converted to fat. Where is most starch stored in plants? What are plastids? What is animal starch called, and where is it produced and stored mainly? What process in animals can be likened to hydrolysis, and what is the main product of hydrolysis? What is that glucose used for? What happens if glucose exceeds energy and storage needs? Another polysaccharide, cellulose, has its glucose units joined together, however, alternating glucose units are 'flipflopped'. Changes in the bond configuration cause changes in the final shape and function of the molecules. Cellulose is found in plant cells, and forms the structurally strong framework in the cell wall. It’s lattice-like structure makes it very strong indeed. • Cellulose is difficult to digest – Cows and other herbivores have microbes in their stomachs to facilitate this process Humans do not! • Chitin, another important structural polysaccharide – Is found in the exoskeleton of arthropods – Can be used as surgical thread CH2O H O OH H H OH H OH H H NH C O CH3 (b) Chitin forms the exoskeleton (a) The structure of the of arthropods. This cicada chitin monomer. is molting, shedding its old exoskeleton and emerging in adult form. (c) Chitin is used to make a strong and flexible surgical thread that decomposes after the wound or incision heals. What is the structural significance of cellulose in plants? How do herbivores digest cellulose? What element can be found in the polysaccharide chitin, that isn’t in other carbohydrates? Fats and oils are made from two kinds of molecules: Complex Structures and Functions of Lipids Do you remember what fats are composed of? • glycerol (a type of alcohol)…and three • fatty acids joined to it by dehydration synthesis. Since there are three fatty acids attached, these are known as triglycerides. The main distinction between fats and oils is whether they’re solid or liquid at room temperature, and this is based on differences in the structures of the fatty acids they contain. The “tail” of a fatty acid is a long hydrocarbon chain, making it hydrophobic. • Hydrophobic means fear of water…so the tail end of a fatty acid is nonpolar, and will not associate with water at all. The “head” of the molecule is a carboxyl group which is hydrophilic. • Hydrophilic means water friendly…so the head end of a fatty acid will readily associate with water. Fatty acids are the main component of soap, where their tails are soluble in oily dirt and their heads are soluble in water to emulsify and wash away the oily dirt. However, when the head end is attached to glycerol to form a fat, that whole molecule is Glycerol hydrophobic. Backbone What process joins the fatty acids to the glycerol to make fat? The tail of a fatty acid is a nonpolar hydrocarbon. What word describes its association with water? The head end of a fatty acid has a carboxyl group which is hydrophyllic. Describe its association with water. What part of the fat makes the whole molecule hydrophobic? • Saturated fatty acids – straight molecules –Generally solid at room temperature –Butter, lard, grease from cooked meats – Contain no double bonds between carbons • Unsaturated fatty acids –Some carbon atoms linked by a “double’ covalent bond –Generally liquid at room temperature –EX: olive oil, fish oils •Hydrogenated vegetable oils contain naturally unsaturated fatty acids that have been saturated artificially (with hydrogen) and are generally solid at room temperature –EX: margarine, vegetable shortening How do you know the fat on this meat is a saturated fat? How are the carbon bonds between saturated and unsaturated fats different? Describe how hydrogenated unsaturated fatty acids are different from natural unsaturated fatty acids. Phospholipids are made from •glycerol •two fatty acids, and (in place of the third fatty acid) •a phosphate group with some other molecule The hydrocarbon tails of the fatty acids are still attached to its other end. hydrophobic, but the phosphate group end of the molecule is hydrophilic because of the oxygens with all of their pairs of unshared electrons. This means that phospholipids are soluble in both water and oil. This becomes especially important as we learn about cells, and the structure of their membranes. Our cell membranes are made mostly of phospholipids arranged in a double layer with the tails of the fatty acids from both layers “inside” (facing toward each other) and the heads facing “out” (toward the watery environment) on both surfaces. This protects the cell from many molecules moving across the membrane. Structure of the Phospholipid Bilayer + CH2 CH2 N(CH3)3 Choline Outside of cell O O O– P Phosphate O CH2 CH O O C O C CH2 Glycerol O Fatty acids Hydrophilic head Hydrophobic tails Inside of cell Figure 5.13 (a) Structural formula (b) Space-filling model (c) Phospholipid symbol How are phospholipids different from other lipids, structurally? What is the significance of the phosphate group on the phospholipid? Why are phospholipids important in the structure of our cell membranes? Cholesterol is not a “bad guy!” Our bodies make about 2 g of cholesterol per day, and that makes up about 85% of blood cholesterol, while only about 15% comes from dietary sources. • Cholesterol is the precursor to our sex hormones and • Vitamin D Vitamin D is formed by the action of UV light in sunlight on cholesterol molecules that have “risen” to near the surface of the skin. Our cell membranes contain a lot of cholesterol (in between the phospholipids) to help keep them “fluid” even when our cells are exposed to cooler temperatures. Steroids may be recognized by their skeleton, consisting of three fused six-membered and one fivemembered ring Waxes are widely distributed in nature. The leaves and fruits of many plants have waxy coatings, which may protect them from dehydration and small predators. The feathers of birds and the fur of some animals have similar coatings which serve as a water repellent. Cuticle Cuticle Ocotillo Cacti Lipoproteins are clusters of proteins and lipids all tangled up together. These act as a means of carrying lipids, including cholesterol, around in our blood. There are two main categories of lipoproteins distinguished by how compact/dense they are. •LDL or low density lipoprotein is the “bad guy,” being associated with deposition of “cholesterol” on the walls of someone’s arteries. •HDL or high density lipoprotein is the “good guy,” being associated with carrying “cholesterol” out of the blood system, and is more dense/more compact than LDL. Many plant pigments, such as anthocyanins, chlorophylls A and B, carotenoids and xanthophylls are lipids. Some lipid pigments can also appear in animals, like cichlids…and people. Xanthophylls and melanins Which is the “bad guy”, LDL or HDL, and why? What lipid plays a key role in the “fluidity” of the cell membranes?