Simulation of PEO - University of the Basque Country
advertisement

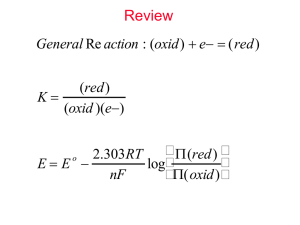
DONOSTIA INTERNATIONAL P HYSICS CENTER Forschungszentrum Jülich Universidad del Pais Vasco MD Simulations of PEO/PMMA blends May 6th 2008 F. Alvarez, A. Arbe, Prof. J. Colmenero M. Brodeck, Prof. D. Richter Motivation What is this all about? • Eurothesis, sponsored by SoftComp, spending time at different SoftComp labs during the PhD San Sebastian and Jülich • MD-simulations of PEO and polymer blends (PEO/PMMA) with very different Tg • Validation of simulations by experiments (PEO) • Analysis of fully atomistic simulations • Rouse mode analysis • Coarse grain simulations? Overview • Introduction – Validation • PEO/PMMA in blend – – – – Structural properties Self-correlation functions and non-Gaussianity Mean Square Displacements Rouse Modes Introduction to the Simulation Setup and Software • Software by • Simulation engine: Discover – Periodic boundary conditions • Densities determined by NPT-simulations • Forcefield: COMPASS – condensed-phase optimized molecular potentials for atomistic simulation studies • Simulation time: 100 ns Δt = 1 fs 100,000,000 steps PEO as Homopolymer Confirmed results Dynamics of individual hydrogen atoms protonated sample* 350K - FOCUS 375K - FOCUS 400K - FOCUS 350K - PI 375K - PI 400K - PI 350K - IN16 350K - Simulation 375K - Simulation 400K - Simulation 10-8 10 w (s) 10 -9 Collective dynamics deuterated sample TOFTOF 1 1.4 1.6 1.8 2 2.2 Q=1.40 Q=1.60 Q=1.80 Q=2.00 Q=2.20 0,8 -10 0,6 10-11 -4 10 -12 10 -13 Q 0,4 0,2 10-14 0 10-15 0,1 1 -1 Q(Å ) 0,01 0,1 1 10 time (ps) *A.-C.Genix, A.Arbe, F.Alvarez, J.Colmenero, L.Willner and D.Richter, Phys.Rev.E, 2005, 72, 031808. *M.Tyagi, A.Arbe, J.Colmenero, B.Frick and J.R.Stewart, Macromolecules, 2006, 39, 3007. 100 PEO as Homopolymer Confirmed results Dynamics of individual hydrogen atoms protonated sample* 10 -8 10 -9 350K - FOCUS 375K - FOCUS 400K - FOCUS 350K - PI 375K - PI 400K - PI 350K - IN16 350K - Simulation 375K - Simulation 400K - Simulation -10 10-11 -4 10 -12 10 -13 10 -14 NSE temperature corrected 1 Temperature: 400K -1 Q=1.3, 1.5 and 1.7 A 0,8 S(Q, t)/S(Q, 0) w (s) 10 Collective dynamics deuterated sample Q 0,6 0,4 0,2 10-15 0 0,1 1 -1 Q(Å ) 0,1 1 10 time (ps) 100 1000 PEO/PMMA in blend Introduction * • Simulating the blend – 5 PEO chains 43 monomers 15 PMMA chains 25 monomers 7170 atoms cell size ~41.5 Å – Density determined by NPT Density 1,12 1,11 1,1 3 Density (g/cm ) • T: 300 (running), 350 and 400 K • 100 ns for each temperature • Huge data files, ~90Gb for 100ns 1,09 1,08 1,07 1,06 1,05 280 * Povray 300 320 340 360 Temperature 380 400 420 PEO/PMMA in blend Structure Scattering dPEO/dPMMA 1 S(Q) 0.5 0 -0.5 0.8 1.6 2.4 3.2 -1 Q (Å ) 4 4.8 5.6 PEO/PMMA in blend Structure Scattering dPEO/dPMMA 1 Total PEO PMMA PEOxPMMA S(Q) 0.5 0 -0.5 0.8 1.6 2.4 3.2 -1 Q (Å ) 4 4.8 5.6 PEO/PMMA in blend Structure Self density Effective density 1 1 PEO PMMA PEO PMMA 0.8 0.8 self 0.6 0.6 effective 0.4 0.2 0.4 0 0 0.2 5 10 distance (Å) 15 20 Composition of the blend (weight): 0 0 5 10 15 distance (Å) 20 25 20% PEO, 80% PMMA PEO/PMMA in blend Comparing the Blend with the Homopolymer PEO in PEO/PMMA 400 K PEO in PEO/PMMA 350 K 0.5 0.5 5 ps 50 ps 500 ps 5000 ps 50000 ps 0.4 4r G (r, t) 0.3 0.3 2 2 s s 4r G (r, t) 0.4 5 ps 50 ps 500 ps 5000 ps 50000 ps 0.2 0.1 0.2 0.1 0 0 0 5 10 Displacement (Å) 15 20 0 5 10 Displacement (Å) 15 20 PEO/PMMA in blend Comparing the Blend with the Homopolymer PEO in PEO/PMMA 350 K non-Gaussianity 0.5 0.4 5 ps 50 ps 500 ps 5000 ps 50000 ps non-Gaussianity 0.3 0.3 2 s 4r G (r, t) 0.4 350 K 400 K 0.35 0.2 0.25 0.2 0.15 0.1 0.1 0.05 0 0 5 10 Displacement (Å) 15 20 0 0.001 0.01 4 3 r (t ) 2 (t ) 1 2 2 5 r (t ) 0.1 1 10 100 time (ps) 1000 10 4 5 10 PEO/PMMA in blend Comparing the Blend with the Homopolymer PEO in PEO/PMMA 350 K non-Gaussianity 0.7 0.4 1 ps 3 ps 1000 ps 0.6 0.3 non-Gaussianity 0.5 0.4 2 s 4r G (r, t) 350 K 400 K 0.35 0.3 0.25 0.2 0.15 0.2 0.1 0.1 0.05 0 0 2 4 6 8 10 0 0.001 0.01 Displacement (Å) 4 3 r (t ) 2 (t ) 1 2 2 5 r (t ) 0.1 1 10 100 time (ps) 1000 10 4 5 10 PEO/PMMA in blend Comparing the Blend with the Homopolymer PEO in PEO/PMMA 400 K non-Gaussianity 0.6 0.4 1 ps 3 ps 1000 ps 0.5 350 K 400 K 0.35 0.3 non-Gaussianity 0.3 2 s 4r G (r, t) 0.4 0.25 0.2 0.15 0.2 0.1 0.1 0.05 0 0 2 4 6 8 10 0 0.001 0.01 Displacement (Å) 4 3 r (t ) 2 (t ) 1 2 2 5 r (t ) 0.1 1 10 100 time (ps) 1000 10 4 5 10 PEO/PMMA in blend Comparing the Blend with the Homopolymer PEO in PEO/PMMA 350 K non-Gaussianity 0.4 0.1 31627 ps 80000 ps 0.3 non-Gaussianity 0.08 0.06 2 s 4r G (r, t) 350 K 400 K 0.35 0.04 0.25 0.2 0.15 0.1 0.02 0.05 0 0 5 10 15 20 Displacement (Å) 25 30 0 0.001 0.01 4 3 r (t ) 2 (t ) 1 2 2 5 r (t ) 0.1 1 10 100 time (ps) 1000 10 4 5 10 PEO/PMMA in blend Comparing the Blend with the Homopolymer PEO in PEO/PMMA 350 K non-Gaussianity 0.1 0.4 31627 ps 39812 ps 50000 ps 63097 ps 80000 ps non-Gaussianity 0.3 0.06 2 s 4r G (r, t) 0.08 350 K 400 K 0.35 0.04 0.25 0.2 0.15 0.1 0.02 0.05 0 0 10 20 30 40 Displacement (Å) 50 60 0 0.001 0.01 4 3 r (t ) 2 (t ) 1 2 2 5 r (t ) 0.1 1 10 100 time (ps) 1000 10 4 5 10 PEO/PMMA in blend Comparing the Blend with the Homopolymer PEO in PEO/PMMA 400 K non-Gaussianity 0.06 0.4 31627 ps 39812 ps 50000 ps 63000 ps 80000 ps 0.05 350 K 400 K 0.35 0.3 non-Gaussianity 0.03 2 s 4r G (r, t) 0.04 0.25 0.2 0.15 0.02 0.1 0.01 0.05 0 0 10 20 30 40 50 60 0 0.001 0.01 Displacement (Å) 4 3 r (t ) 2 (t ) 1 2 2 5 r (t ) 0.1 1 10 100 time (ps) 1000 10 4 5 10 PEO/PMMA in blend MSD of PEO-hydrogens Displacement Histograms Comparison of homopolymer/blend at t=50 ns Mean Square Displacement 10 0.1 homopolymer - 350 K blend - 350 K homopolymer - 400 K blend - 400 K 0.08 blend - 350 K blend - 400 K homopolymer - 350 K homopolymer - 400 K 1000 Slopes: t^0.36 t^0.41 t^0.51 t^0.52 100 0.04 2 s 2 MSD (Å ) 0.06 4r G (r, t) 4 10 0.02 1 0 0.1 -0.02 0 10 20 30 40 Displacement (Å) 50 60 0.01 0.01 0.1 1 10 100 time (ps) 1000 Movement of PEO atoms restricted by the rather stiff PMMA-matrix 10 4 10 5 PEO/PMMA in blend MSD of hydrogen-atoms PEO/PMMA at 350 K Methyl-group H H C Main-chain 1000 100 H C H 10 2 C MSD (Å ) H PEO - 350 K PMMA-Main - 350 K PMMA-Methyl - 350 K PMMA-Ester - 350 K H H H H C 25 C O 1 O H Ester-group Complex, stiff structure 0.1 0.01 0.01 0.1 1 10 100 time (ps) 1000 10 4 10 5 PEO/PMMA in blend Comparing the MSD for two temperatures PEO/PMMA at 350 and 400 K 10 2 MSD (Å ) 1000 PEO - 350 K PEO - 400 K PMMA-Main - 350 K PMMA-Main - 400 K 100 PMMA-Methyl - 350 K PMMA-Methyl - 400 K PMMA-Ester - 350 K PMMA-Ester - 400 K 1 0.1 0.01 0.01 0.1 1 10 100 time (ps) 1000 10 4 10 5 PEO/PMMA in blend Forming Blobs PEO/PMMA in blend Results – Rouse Modes Rouse modes blend 400 K 1 We investigate the behavior of PEO in the blend! 0.8 0.6 1 p n X p (t ) dn cos( ) rn (t ) N 0 N 0.4 Fourier transformation of blob-coordinates 0.2 Nl 2 X p (t ) X p (0) 2 2 exp(t / p ) 6 p pp (t) N 0 1 10 100 1000 time (ps) 10 4 10 5 (t ) Ae ( t /WW ) PEO/PMMA in blend Results – Rouse Modes stretching parameter relaxation time <> 10 p 1 107 Blend - 350K Blend - 400K Blend - 350K Blend - 400K 6 -2.75 0,9 p 105 -3.87 4 p p -2.50 p 10 <> (ps) 0,8 0,7 1000 0,6 100 0,5 10 -2.97 p 1 0,4 1 10 p 0 10 30 20 p Nl 2 X p (t ) X p (0) 2 2 exp((t / ) ) 6 p 40 PEO/PMMA in blend MSD of hydrogen-atoms PEO/PMMA at 350 K Methyl-group H H C Main-chain 1000 100 H C Rouse regime H 10 2 C MSD (Å ) H PEO - 350 K PMMA-Main - 350 K PMMA-Methyl - 350 K PMMA-Ester - 350 K H H H H C 25 C O 1 O H Ester-group Complex, stiff structure 0.1 0.01 0.01 0.1 1 10 100 time (ps) 1000 10 4 10 5 PEO/PMMA in blend MSD of hydrogen-atoms PEO/PMMA at 400 K Methyl-group H H C Main-chain 1000 H 100 H C H H H C Rouse regime H 10 2 C MSD (Å ) H PEO - 400 K PMMA-Main - 400 K PMMA-Methyl - 400 K PMMA-Ester - 400 K 25 C O 1 O H Ester-group Complex, stiff structure 0.1 0.01 0.01 0.1 1 10 100 time (ps) 1000 10 4 10 5 PEO/PMMA in blend Results – Rouse Modes stretching parameter relaxation time <> 10 p 1 107 Blend - 350K Blend - 400K Blend - 350K Blend - 400K 6 -2.75 0,9 p 105 -3.87 4 p p -2.50 p 10 <> (ps) 0,8 0,7 1000 0,6 100 0,5 10 -2.97 p 1 0,4 1 10 p 0 10 30 20 p Nl 2 X p (t ) X p (0) 2 2 exp((t / ) ) 6 p 40 PEO/PMMA in blend Results – Rouse Modes stretching parameter relaxation time <> 10 10 7 chain chain chain chain chain chain chain chain chain chain 6 chain 1 chain 2 chain 3 chain 4 chain 5 chain 1 chain 2 chain 3 chain 4 chain 5 0,9 0,8 p 4 1 2 3 4 5 1 2 3 4 5 <> (ps) 105 10 p 1 0,7 1000 0,6 100 0,5 10 1 0,4 10 1 0 10 p 20 30 p Nl 2 X p (t ) X p (0) 2 2 exp((t / ) ) 6 p 40 PEO/PMMA in blend Results – Rouse Modes stretching parameter relaxation time <> 10 7 10 6 p 1 Homopolymer - 350K Homopolymer - 400K Blend - 350K Blend - 400K Homopolymer - 350K Homopolymer - 400K Blend - 350K Blend - 400K 0,9 105 4 p 10 <> (ps) 0,8 0,7 1000 0,6 100 0,5 10 0,4 1 1 10 0 10 p 20 30 p Nl 2 X p (t ) X p (0) 2 2 exp((t / ) ) 6 p 40 Conclusion • Simulation of homopolymer validated by various experimental techniques • Simulation of the blend: extraction of valuable information from full trajectories • Development of a second peak for high Δt Caging-effect or simulation artifact? • Formation of blobs Rouse mode analysis Thank you… • Softcomp Eurothesis Project • DIPC, University of the Basque Country San Sebastian – J. Colmenero – F. Alvarez – A. Arbe • Forschungszentrum Jülich – D. Richter relaxation time (corrected) --10 5 10 4 Blend - 350K and 400K 1mon and 3mon 1000 100 PEOPMMA13 - 1mon PEOPMMA9 - 1mon PEOPMMA13 - 3mon PEOPMMA9 - 3mon 10 1 1 10 rouse - mode --- relaxation time (corrected) Homopolymer - 350K and 400K 1mon and 3mon 10 5 10 4 1000 100 PEO28 - 1mon PEO30 - 1mon PEO28 - 3mon PEO30 - 3mon 10 1 1 10 rouse - mode --MSD PEO 104 1000 Center of mass 3 blobs per momoner 1 blob per monomer PEO (pure) 0.52 100 10 MSD 0.65 0.8 1 0.61 0,1 0,01 0,001 0,0001 0,01 0,1 1 10 100 time(ps) 1000 4 10 5 10 PEO as Homopolymer Coarse Graining PEO • Micro – Meso mapping – Simplest idea: 1 monomer = 1 blob – Obtain probabilities from MD simulations • Bond distance • Bond angle • … – Assume that the probability distributions factorize P(l , ,...) P(l ) P( ) P(...) – Boltzmann factors of interaction potentials U l (l ) P(l ) exp( ) k BT PEO as Homopolymer Coarse Graining PEO • Micro – Meso mapping – Determine potentials and forces U l (l ) ln P(l ) d F (l ) ln P (l ) dl – – – – Repeat for different temperatures Compile into tables (no analytical form necessary) Write software (or use free open source alternative) LAMMPS – Large-scale Atomic/Molecular Massively Parallel Simulator, lammps.sandia.gov PEO as Homopolymer Results – Coarse Graining Distance between blobs Angle between blobs 2 0,015 1,5 probability probability 0,01 1 0,005 0,5 0 0 2 2,5 3 distance (Å) 3,5 4 0 50 100 angle • From these probabilities we can calculate an effective potential 150 PEO as Homopolymer Coarse Graining – Effective Potentials Distance between blobs Angle between blobs 2 0,015 1,5 probability 1 0,005 0,5 0 0 2,5 3 distance (Å) 3,5 4 0 Distance between blobs 50 100 angle 150 Angle between blobs 8 8 7,5 6 7 6,5 4 Energy 2 Energy probability 0,01 6 5,5 2 5 0 4,5 4 -2 0 2 2,5 3 distance (Å) 3,5 50 100 4 angle 150 200 PEO as Homopolymer Coarse Graining – Non-bond interactions Probability to find another blob Non-bond interactions Non-bond potential 4 1,5 3 1 Energy probability 2 1 0,5 0 0 -1 0 2 4 8 6 Distance (Å) 10 12 14 0 2 4 6 8 Distance (Å) 10 12 14