6. Neutron chain reaction systems_BNEN_Intro_2015
advertisement

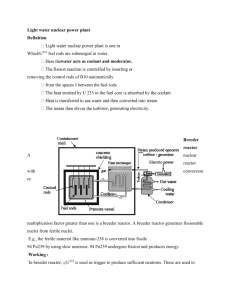
Neutron Chain Reaction Systems William D’haeseleer Neutron Chain Reaction Systems References: • Lamarsh, NRT, chapter 4 • Lamarsh & Baratta, chapter 4 • Also Duderstadt & Hamilton § 3.I Concept of chain reaction • Initially, reactor contains a certain amount of fuel, with initially Nf(0) fissile nuclei (e.g. U-235) • To get fission process started necessary to have an “external” neutron source → this source initiates fission process Concept of chain reaction U 235 92 1 0 n X Y 2.5 n • The by fission produced neutrons can be absorbed in U-235 → can lead to fission 2.5 n fission 2.5 n etc… etc… CHAIN REACTION Concept of chain reaction Chain reaction 235 U Concept of chain reaction Concept of chain reaction • If “few” neutrons leak out, or parasitically absorbed: → exponentially run-away chain reaction super critical reactor k>1 • If “too many” neutrons leak out, or parasitically absorbed: → exponentially dying-out chain reaction sub critical reactor k<1 Concept of chain reaction • If after one generation precisely 1 neutron remains, which “activates” again precisely 1 neutron, → stationary regime critical reactor k=1 k = multiplication factor number of neutrons in one generation = number of neutrons in previous generation Concept of chain reaction must be k=1 Multiplication factor 1. Infinite reactor (homogeneous mixture of enriched U and moderator) • • • Assume at a particular moment n thermal neutrons absorbed in fuel These produce n η v f n fission neutrons a But sometimes also fissions due to fast neutrons → correction factor ε ≥ 1 (e.g., 1.03) in fact n η ε fission neutrons Multiplication factor • These n η ε neutrons must be slowed down to thermal energies p ≡ resonance escape probability = probability for not being absorbed in any of the resonances during slowing down nηεp thermalized neutrons • After thermalization, a fraction f will be absorbed in the fuel U-235; the remainder absorbs in structural material, moderator material, U-238, etc nηεpf thermal neutrons absorbed in the fuel Multiplication factor • Hence, after the next generation: n p f k f p n k multiplication factor in medium Multiplication factor Note: three-step approach for multiplication factor → mono-energetic infinite reactor → moderation in infinite thermal reactor → moderation in finite thermal reactor Multiplication factor i. Mono-energetic infinite reactor Multiplication factor i. Mono-energetic infinite reactor PAF = prob that neutron will be absorbed in the fuel F a F a remainder a f a F a “thermal utilization factor” Multiplication factor i. Mono-energetic infinite reactor Multiplication factor Pf = prob that an absorbed neutron in the fuel leads to fission PF v F f F a F f F a Number of neutrons in next generation: N 2 vPf PAF N1 fN1 v N2 k f N1 a F f Multiplication factor ii. Moderation in infinite thermal reactor Now η identified with absorption of thermal neutrons Also f defined for thermal neutrons → reasons for name “thermal utilization factor” total number of fission numbers Define = number of fission neutrons caused by thermal neutrons Define p = resonance escape probability k f p "four factor formula" Multiplication factor iii. Moderation in finite thermal reactor Multiplication factor iii. Moderation in finite thermal reactor PNL= non-leakage probability k ≡ keff = k∞ PNL k = multiplication factor for finite reactor Multiplication factor 2. Finite reactor keff k PNL non leakage probability A critical reactor always has keff = 1 Influencing factors of keff : - leakage probability : geometry - amount of fuel: composition - presence/absence strong absorbers: composition Critical Mass • The larger the surface of a certain volume, the higher the probability to leak away 4 R3 volume R e.g., for sphere: 3 2 R surface 4 R 3 • The larger R: – more fissile isotopes in volume – larger leak-through surface But Vol ∕ Surf → relatively more production of neutrons than leakage Critical Mass • Critical mass = minimal mass for a stationary fission regime • Examples: critical mass of U-235 ≤ 1 kg -when homogeneously dissolved as uranium salt in H2O -when concentration of U-235 > 90% in the uranium salt ≥ 200 kg -when U-235 is present in 30 tonnes of natural uranium embedded in matrix of C ! Natural uranium alone with 0.7% U-235 can never become critical, whatever the mass (because of absorption in U-238) Critical Mass Critical Mass Critical Mass Nuclear Fuels * fissile isotopes * fertile isotopes U-233 U-235 Pu239 Th-232 U-238 only this isotope is available in nature U-233 Pu-239 U-235 cannot be made artificially → to increase fraction of U-235 in a “U-mixture” → need to ENRICH “enrichment” Nuclear Fuels * consider reactor with 97% U-238 and 3% U-235 most of the U-235 fissions, “produces” energy, produces n U-238 absorbs neutrons Pu-239 an amount Pu-239 fissions…..energy…..n….. an amount Pu-239 absorbs n → Pu-240 … Pu-241 … Pu-242 an amount Pu-239 remains behind Production of Pu isotopes Evolution of 235U content and Pu isotopes in typical LWR Production of Pu isotopes Nuclear Fuels Conversion factor C # of fissile isotopes formed # of fissile isotopes "consumed" by fission or absorption Nuclear Fuels * In a U-235 / U-238 reactor, Pu-239 production consumption of N U-235 atoms → NC Pu-239 atoms produced * In a Pu-239 / U-238 reactor, Pu-239 production consumption of N Pu-239 atoms →NC Pu atoms produced →(NC)C Pu atoms produced → (NC²)C Pu atoms produced →etc. NC 2 3 NC NC NC 1 C Nuclear Fuels * C<1 C>1 convertor breeder reactor * η>1 for criticality write η = 1+ ζ To be used for “conversion” (in addition to leakage, parasitary absorption) Nuclear Fuels f v v a 1 η(E) for U-233, U-235, Pu-239 & Pu-241 Ref: Duderstadt & Hamilton Slowing down (“moderation”) of neutrons • Fission neutrons are born with <E> ~ 2 MeV • Fission cross section largest at low E (0.025 eV) • →need to slow down neutrons as quickly as possible = “ moderation” • Mostly through elastic collisions (cf. billiard balls) Slowing down (“moderation”) of neutrons • Best moderator materials: → mass moderator as low as possible 1 1 H is the perfect moderator : m 1 1 H m n 1 0 → moderator preferably low neutron-absorption cross section Slowing down (“moderation”) of neutrons Hence: * H2O -good moderator (contains much 11 H) -but absorbs considerable amount of neutrons →U to be enriched -can also serve as coolant * D2O -still small mass: good moderator -absorbs fewer n than H2O →can operate with natural U: CANDU -can also serve as coolant Slowing down (“moderation”) of neutrons * 12 6 C graphite: -now need for separate cooling medium → other properties of moderator materials -good heat-transfer properties -stable w.r.t. heat and radiation -chemically neutral w.r.t. other reactor materials Slowing down (“moderation”) of neutrons • Time to “thermalize” from ~ 2 MeV → 0.025 eV in H2O: tmod ~ 1 μs tdiff ~ 200 μs = 2 x 10-4 s time that a neutron, after having slowed down, will continue to “random walk” before being absorbed. tgeneration ~ 2 x 10-4 s Reflector To reduce the leakage of neutrons out of reactor core → surround reactor core with “n-reflecting” material Usually, reflector material identical to moderator material Note: There exist also so-called “fast” reactors But most commercial reactors are “thermal” reactors (=reactors with thermal neutrons)