Naming Ionic Compoun..
advertisement

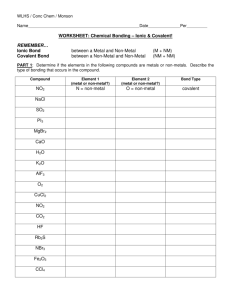
Here we’ll go over a couple of examples of naming simple ionic compounds containing 2 elements. Binary Compounds -Compounds containing only 2 elements The prefix “bi” means 2, so compounds containing only two elements are called binary compounds. Binary Compounds -Compounds containing only 2 elements Binary Ionic Compounds -Contain a metal and a non-metal Binary Ionic compounds are made up of a metal and a non-metal Metals Non -Metals This Periodic Table has a staircase drawn with a black line. The elements to the left of the staircase are metals and those to the right are non-metals. Charge on the most common ion of this element When an atom of a metal loses one or more electrons, it forms a positive ion. It is the ions of metals that form ionic compounds. Charge on the most common ion of this element The little number on the top right of an element’s box is the charge of the most common ion of the element. We see that magnesium has only one number so it only has 1 common charge. Charges on the most common ions of this element Some metals, like iron form ions with more than one common charge. Charges on the most common ions of this element One common ion of iron has a positive 3 charge and the other common ion has a positive 2 charge. Metals with Only One Common Charge At this point, we’ll discuss only compounds of metals with a single charge. These are shaded in green here. We’ll discuss compounds formed by the other metals in a later video. Rules for Naming Binary Ionic Compounds in which the Metal has Only One Charge 1. Always name the metal first. 2. Name the non-metal second. 3. Change the ending of the non- Here we’ll go over the basic rules for naming a binary, or 2 element, ionic compound in which the metal present has only one possible charge. Rules for Naming Binary Ionic Compounds in which the Metal Compound has Only One Charge names are all in lower case 1. Always name the metal first. 2. Name the non-metal second. 3. Change the ending of the nonmetal so it ends in -ide. Just a general comment about compound names. Compound names are always written in lower case and not capitalized, unless they are at the beginning of a sentence. Rules for Naming Binary Ionic Compounds in which the Metal Compound has Only One Charge names are all in lower case 1. Always name the metal first. 2. Name the non-metal second. 3. Change the ending of the nonmetal so it ends in -ide. Always name the metal first. The metal name is not changed in any way. If it’s a metal with only one possible charge, we don’t need to worry about any numbers that are in a given formula. Rules for Naming Binary Ionic Compounds in which the Metal Compound has Only One Charge names are all in lower case 1. Always name the metal first. 2. Name the non-metal second. 3. Change the ending of the nonmetal so it ends in -ide. The name of the non-metal always comes second Rules for Naming Binary Ionic Compounds in which the Compound Metal are all has Only One Charge innames lower case 1. Always name the metal first. 2. Name the non-metal second. 3. Change the ending of the non- The name of the non-metal must be changed so that it ends in the letters I D E. For example chlorine is changed to chloride and oxygen is changed to oxide. 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. A compound is made up of the elements potassium and sulphur. Name this compound. potassium sulph ur Here’s an example question. We’re told that a compound is made up of the elements potassium and sulphur and we’re asked to name this compound. Metals Non -Metals A compound is made up of the elements potassium and sulphur. Name this compound. We start by locating potassium and sulphur on the periodic table. We see that potassium is a metal and sulphur is a non-metal, so this is an ionic compound. One possible charge A compound is made up of the elements potassium and sulphur. Name this compound. Looking more closely at potassium, we see that it has only one possible charge. Therefore, we can use the simple method of naming outlined here. 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. potassium sulph u r A compound is made up of the elements potassium and sulphur. Name this compound. We write the metal name first, potassium, the same way it is spelled on the periodic table. Notice we did not capitalize it. 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. potassium sulph u r A compound is made up of the elements potassium and sulphur. Name this compound. Now we note that the second element, the non-metal, is sulphur 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. potassium sulph u r A compound is made up of the elements potassium and sulphur. Name this compound. The third step says, we must change the non-metal name so that it ends in the letters IDE. Notice it now ends in the letters U-R 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. potassium sulph u ide r A compound is made up of the elements potassium and sulphur. Name this compound. So we drop the ending “ur” and replace it with “ide”. 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. potassium sulph ide A compound is made up of the elements potassium and sulphur. Name this compound. Giving us the name potassium sulphide Name 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. potassium ide sulph or potassium sulf ide Name A compound is made up of the elements potassium and sulphur. Name this compound. In many places sulphur is spelled with an “f” rather than a “ph”, so the spelling s-u-l-f-i-d-e is also considered correct in the name potassium sulphide. 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. The formula for a compound is Cd3P2. Name this compound. potassium sulph ur Here’s another example question. We’re told that the formula for a compound is Cd3P2 and we’re asked to name this compound. Metals Non -Metals The formula for a compound is Cd3P2. Name this compound. We start by locating Cd and P on the periodic table. We see that they are the metal cadmium and the non-metal phosphorus, so this is an ionic compound. One possible charge The formula for a compound is Cd3P2. Name this compound. Looking more closely at cadmiun, we see that it has only one possible charge. Therefore, we can use the simple method of naming outlined here. 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. cadmium phosph orous The formula for a compound is Cd3P2. Name this compound. We write the name of the metal first, unchanged. It is cadmium 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. cadmium phosph orus The formula for a compound is Cd3P2. Name this compound. Next, we note the name of the non-metal, which is phosphorus 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. cadmium phosph orus The formula for a compound is Cd3P2. Name this compound. However, rule number 3 tells us that the name of the non-metal must end in the letters I-D-E. 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. cadmium phosph ide orus The formula for a compound is Cd3P2. Name this compound. So we take the ending o-r-u-s (click) are we replace it with i-d-e 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. cadmium phosph ide Name The formula for a compound is Cd3P2. Name this compound. Giving us the correct name for this compound, cadmium phosphide. 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. Cd3P2 subscripts have no effect on the name subscripts cadmium phosph ide The formula for a compound is Cd3P2. Name this compound. Notice that the formula in this example has numbered subscripts 3 and 2 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. Cd3P2 subscripts have no effect on the name subscripts cadmium phosph ide The formula for a compound is Cd3P2. Name this compound. But because the metal in this compound, cadmium only has one possible charge 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. Cd3P2 subscripts have no effect on the name subscripts cadmium phosph The formula for a compound is Cd3P2. Name this compound. The subscripts have no effect on the name. ide 1. 2. 3. Always name the metal first. Name the non-metal second. Change the ending of the non-metal so it ends in -ide. Cd3P2 subscripts have no effect on the name subscripts cadmium phosph The formula for a compound is Cd3P2. Name this compound. And the name is simply cadmium phosphide ide