chem compounds unit homework - wbm
advertisement

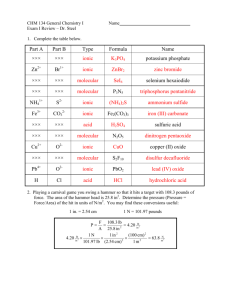
CHEMISTRY Chemical Compounds Unit Day 1: Objectives 6.1.1, 6.1.2, 6.1.3, 6.1.4 Before class: Read section 6-1 Answer the following. Complete sentences are not needed. 1. Define: a. chemical bond, b. ionic bonding, c. covalent bonding, d. nonpolar-covalent bond, e. polar-covalent bond After class: Write the letter of the best answer. 1. A large difference in electronegativity between two atoms in a bond results in a. polar covalent bonding b. nonpolar covalent bonding c. ionic bonding d. repulsion between two atoms 2. Which of the following happens when atoms bond with each other? a. The atoms increase in potential energy. b. The potential energy of the compound is maximized. c. Their electronegativity dramatically incrases. d. They create arrangements of matter that are more stable. Element Electronegativity Element Electronegativity Hydrogen 2.1 Oxygen 3.5 Sodium 0.9 Carbon 2.5 Calcium 1.0 Cobalt 1.8 Lithium 1.0 Nitrogen 3.0 3. Use the electronegativity values above to classify the bonds that would form between atoms of the following elements as ionic, polar-covalent, or nonpolar-covalent a. H and O, b. H and C, c. Na and Co, d. Li and N, e. Ca and H, f. N and O, g. Li and Ca, h. N and Ca, i. Na and O, C and O Day 2: Objectives 6.2.1, 6.2.2 Before class: Read pages 164 – 170 Answer the following. Complete sentences are not needed. 1. Define: a. molecule, b. molecular compound, c. chemical formula, d. molecular formula, e. diatomic molecule, f. bond length, g. bond energy, h. octet rule, i. electron-dot notation After class: Write the letter of the best answer. 1. The molecular formula H2O indicates a molecule a. with two atoms of oxygen bonded with one atom of hydrogen. b. with two atoms of hydrogen bonded with one atom of oxygen. c. that includes only ionic bonds. d. that is diatomic. CHEMISTRY Chemical Compounds Unit 2. Which statement is true? a. A molecule can exist only if two of the same type of atom are bonded. b. The strength of attraction between charged particles depends only on the type of atom involved in the bond. c. An atom of any element is a unit that can stand on its own. d. The strength of attraction between charged particles depends on the distance between the atoms involved in the bond. 3. Noble-gas atoms are able to exist independently in nature because a. they are exceptions to the octet rule. b. their bond energies are low compared to their bond lengths. c. their electron configurations are more stable that those of other atoms. d. they share electrons in overlapping orbitals with other noble-gas atoms. 4. Which statement is true? a. As atoms are drawn together, the potential energy decreases. b. Potential energy is at a maximum when attractive forces are balanced by repulsive forces. c. The composition of an element is given by its molecular formula. d. Hydrogen is the only element that bonds to achieve noble-gas configurations 5. Use Table 6-1 to create a graph comparing bond length to bond energy. Plot bond length on the x-axis in units of pm and bond energy on the y-axis in unts of kJ/mol. Remember to give your graph a title and label your axes. 6. What does your graph (from 8.) show about the relationship between bond length and bond energy? Day 3: Objectives 6.2.3, 6.2.4 Before class: Read pages 170 – 175 Answer the following. Complete sentences are not needed. 1. Define: a. unshared pair, b. Lewis structure, c. structural formula, d. single bond, e. double bond, f. triple bond, g. multiple bonds, h. resonance CHEMISTRY Chemical Compounds Unit After class: Write the letter of the best answer. 1. Which of the following statements is true? e. A pair of dots in electron-dot notation always represents an electron pair of a lone atom in a compound. f. Only valence electrons of a particular atom are shown in electron-dot notation. g. A single bond is formed when a single electron brings two atoms together. h. Covalent bond formation involves the electrons closest to the nuclei of the atoms. 2. Draw Lewis structures of each of the following: a. CH4O, b. CH4, c. CS2, d. HCN, e. NH3, f. SOCl2 (S is the central atom) Day 4: Objectives 6.3.1,6.3.2, 6.3.3, 6.3.4 Before class: Read section 6-3 Answer the following. Complete sentences are not needed. 1. Define: a. ionic compound, b. formula unit, c. lattice energy, d. polyatomic ion After class: Write the letter of the best answer. 1. Which of the following statements is true of molecular compounds? a. When dissolved in water, they conduct electricity. b. They are hard, brittle substances. c. Their lattice energies are positive rather than negative. d. Some are completely gaseous at room temperature. 2. Which statement is true of ionic compounds? a. The distances between the ions vary throughout the crystal. b. All ionic crystals have the same structure. c. The ions cannot move in the solid state. d. All ionic compounds are able to dissolve in water. 3. Which statement is true? a. Calculating lattice energies helps chemists compare bond strengths in ionic compounds. b. Table salt is a molecular compound. c. Na2Cl2 is a formula unit. d. Ionic crystals for, so that the potential energy of a compound is minimized. CHEMISTRY Chemical Compounds Unit 4. Which statement is true? a. Molecular formulas represent the simplest formulas for compounds. b. The forces holding ions together in ionic bonds are relatively weak. c. Intermolecular attractions that hold molecules together are stronger than ionic attraction. d. A polyatomic ion with a shortage of electrons has a positive charge. 5. Each of the following compounds is either ionic or molecular. Based on the information given, write ionic if the compound is more likely to be ionic or write molecular if it is more likely to be molecular a. Compound A is a gas at room temperature. b. Compound B breaks easily. c. Compound C conducts electricity when dissolved in water. d. Compound D has a value for lattice energy. e. Compound E has a low melting point. f. Compound F is not soluble in water. g. The components of compound G are held together by electrical attraction. Day 5: Objectives 6.4.1, 6.4.2, 6.4.3 Before class: Read section 6-3 Answer the following. Complete sentences are not needed. 1. Define: a. metallic bonding, b. malleability, c. ductility After class: Write the letter of the best answer. 1. Why are metals able to conduct electricity? a. The valence electrons that make up a metal are highly mobile. b. Electrons are bound to individual ions that are held in place in metallic crystal structures. c. Metals are shiny and malleable. d. The shortage of electrons in metals gives them a positive electrical charge. 2. A blacksmith can shape metal into a horseshoe. This shows that the metals is a. ductile b. a good conductor of electricity c. malleable d. a liquid at room temperature CHEMISTRY Chemical Compounds Unit 3. Create the Venn diagram to compare and contrast metals and ionic compounds. In the overlapping area, note how the two substances are similar. In the individual areas, list qualities pertaining only to that particular substance. Ionic Compounds Metals M at h Com poser 1. 1. 5 ht t p: / / www. m at hcom poser . com Day 6: Conductivity Lab Day 7: Objectives 6.5.1, 6.5.2 Before class: Read pages 183 - 187 Answer the following. Complete sentences are not needed. 1. Define: a. molecular polarity, b. VSEPR theory After class: Write the letter of the best answer. 1. Which is true of an AB4 molecule? a. The electron pairs separate to form a tetrahedron. b. The molecule contains hydrogen. c. The molecule has ionic bonds. d. The molecule has both single and double bonds. 2. A molecule with 2 atoms bonded to the central atom generally has which shape? a. linear b. bent c. trigonal planar d. either a or b 3. Create a flow chart or an outline to show the steps in using VSEPR theory to predict molecular geometry of a compound. Consider the use of Lewis structure in valence electrons as you create your chart or outline. 4. Illustrate the steps in your chart or outline as you use VSEPR theory to predict the molecular geometry of Cl2O. Day 8: Objectives 6.5.3, 6.5.4, 6.5.5 Before class: Read pages 187 – 193 Answer the following. Complete sentences are not needed. 1. Define: a. hybridization, b. hybrid orbitals, c. intermolecular forces, d. dipole, e.dipole-dipole forces, f. hydrogen bonding, g. London dispersion forces CHEMISTRY Chemical Compounds Unit After class: Write the letter of the best answer. 1. The higher the boiling point of a substance a. the weaker the force between particles b. the stronger the force between particles c. the more likely it is the substance is nonpolar-covalent d. the more likely it is that the substances is polar-covalent 2. In general, intermolecular forces are a. stronger than bonds that join atoms in molecules b. weaker than bonds that join atoms in molecules, but stronger than ionic bonds c. stronger than bonds that join metal atoms in solid metals d. weaker than bonds that join atoms in molecules and ions in ionic compounds 3. Which statement is true? a. London forces explain the high boiling point of water. b. Individual bond dipoles are contained only in ionic solutions. c. Hydrogen bonding explains the high boiling point of ammonia. d. Intermolecular forces are the forces of attraction between atoms 4. Hydrogen bonding is a special type of a. sp3 orbital b. London dispersion force c. dipole-dipole force d. molecular geometry Complete the following sentences. 5. Hydrogen bonding is responsible for __________________ of water. 6. London forces are the only intermolecular forces that act upon_____________________. 7. As atomic or molar masses increase, London forces _________________. Answer the following using complete sentences. 8. How can a polar molecule induce a dipole in a nonpolar molecule? 9. In what direction does current in a dipole flow? Days 9 and 10: Molecular Geometry Lab Day 11: Objectives 7.1.1, 7.1.2, 7.1.3 Before class: Read pages 203 - 211 Answer the following. Complete sentences are not needed. 1. Define: a. monatomic ions, b. binary compounds, c. nomenclature, d. oxyanions CHEMISTRY Chemical Compounds Unit After class: Write the letter of the best answer. 1. Which statement is true? a. All main-group elements are able to form ions b. The total numbers of positive charges and negative charges must be equal in a binary compound. c. The nonmetals of groups 15, 16, and 17 lose electrons to form cations. d. Elements in the d block form ions of only one charge. 2. Why do many main-group elements lose or gain electrons to form ions? a. The elements are unstable and therefore lose electrons. b. Their tendency is to maximize potential energy. c. The elements form covalent bonds, sharing their electrons with other atoms. d. Their tendency is to form a complete outermost octet in a noblegas configuration. 3. Which is the correct formula for a compound made of Sn2+ and NO3– ? a. Sn2NO3 b. Sn(NO3)2 c. Sn3NO2 d. 2Sn3NO 4. Which is the correct formula for silver chloride? a. Ag+Cl– b. 2Ag2Cl c. AgClO d. AgCl Answer the following using complete sentences. 5. Explain what each chemical formula tells you about the composition of the compound. The first one has been done for you. a. NaNO3 The subscript 3 refers to the number of oxygen atoms. There are one atom of sodium and one atom of nitrogen for every three atoms of oxygen. b. AgCl c. Ba(OH)2 d. (NH4)2SO4 Matching 6. Br – ________ a. nitrate – ___________ 7. OH b. chlorite – 8. NO2 _______ c. hydroxide 9. NO3 – _______ d. bromide – 10. O2 ________ e. nitrite 11. CN – _______ f. ammonium – 12. ClO2 ______ g. oxide + 13. NH4 _______ h. cyanide CHEMISTRY Chemical Compounds Unit 14. Copy and complete the chart by supplying the missing information Name of Compound or Ion Formula of compound or ion Copper (II) ion NH4+ Bismuth (III) bromide Tin(II) fluoride MgF2 Iron (III) cyanide S2 – Potassium permanganate Day 12: Objectives 7.1.2, 7.1.3, 7.1.4, 7.1.5 Before class: Read pages 211 - 215 Answer the following. Complete sentences are not needed. 1. Write the name, formula, and charge for each of the following: a. acetate, b. ammonium, c. chlorate, d. chlorite, e. hydroxide, f. hypochlorite, g. nitrate, h. nitrite, i. perchlorate, j. permanganate, k. carbonate, l. peroxide, m. sulfate, n. sulfite, m. phosphate After class: Write the letter of the best answer. 1. Which is the correct formula for phosphorus pentafluoride? a. P5F b. PFl5 c. PF5 d. PF 2. NaF6 is the formula for which compound? a. sodium hexafluoride b. sodium pentafluoride c. hexafluoride sodium d. hexasodium fluoride 3. Which of the following is the formula for silicon dioxide? a. S2O b. Si2O c. SO2 d. SiO2 4. H2SO3 is the formula for what compound? a. sulfuric acid b. sulfurous acid c. dihydrous sulfuric acid d. hydrosulfuric acid CHEMISTRY Chemical Compounds Unit 15. Copy and complete the charts by supplying the missing information. Try not to use your book. Number 1 2 3 4 5 6 7 8 9 10 Prefix Name of Compound Formula of Compound CO2 Nitrogen dioxide S2Cl2 Carbon Tetrachloride P4O7 SF6 Ice Diphosphorus pentoxide Day 13: Chemistry Bingo Day 14: Objectives 7.2.1, 7.2.2, 7.2.3 Before class: Read section 7 – 2 Answer the following. Complete sentences are not needed. 2. Define: a. oxidation numbers After class: Write the letter of the best answer. 1. What are the oxidation numbers for the atoms in AlCl3 a. +3, -1 b. +3, +1 c. -3, -1 d. -3, +1 CHEMISTRY Chemical Compounds Unit 2. In a ClO4 – ion, what is the oxidation number of Cl? a. +4 b. -4 c. +5 d. +7 3. What is the Stock system name of SO2? a. sulfur dioxide b. sulfur trioxide c. sulfur(IV) oxide d. sulfur(II) oxide 4. HNO2 is the formula for which compound? a. huydrogen nitrogen dioxide b. nitrogen(II) dioxide c. nitrous acid d. ammonium(II) oxide 16. Copy and complete the chart by supplying the missing information Stock system name ChemicalFormula Prefix system name Carbon(IV) iodide Sulfur(VI) oxide Arsenic(III) sulfide Nitrogen(III) chloride Phosphorus (V) chloride Water Lead(IV) oxide Nitrogen(I) chloride Day 15: Objective 7.3.1 Before class: Read pages 221 – 224 Answer the following. Complete sentences are not needed. 1. Define: a. formula mass, b. molar mass After class: Write the letter of the best answer. 1. In the compound Al2(SO4)3 there are a. 2 mol Al, 3 mol S, 12 mol O b. 2 mol Al, 4 mol S, 4 mol O c. 6 mol Al, 3 mol S, 12 mol O d. 2 mol Al, 3 mol S, 7 mol O CHEMISTRY Chemical Compounds Unit Complete the charts by supplying the missing information Compound Number of moles of atoms of each element in one mole of the compound CO2 1 mol C, 2 mol O AlCl3 Cu2O Ba(SCN)2 LiH H2SO4 Compound CO2 SnI4 NaH2PO4 Hg2Cl2 Ca(C2H3O2)2 molar mass Day 16: Objective 7.3.2, 7.3.3, 7.3.4 Before class: Read pages 224 – 228 Answer the following. Complete sentences are not needed. 3. Define: a. percentage composition 1. Example: What is the mass in grams of 3.04 mol of ammonia vapor, NH3? 2. You try: what is the mass in grams of 0.257 mol of calcium nitrate, Ca(NO3)2? 3. Example: how many moles of SO2 are in 3.82 g? 4. You try: how many moles of Cl2 are there in 77.1 g? 5. Example: How many molecules are there in 77.1 g of Cl2? 6. You try: How many molecules are in 4.15 x 10-3 g of C6H12O6? 7. Example: Find the percentage composition of sodium nitrate, NaNO3. 8. You try: find the percentage composition of silver sulfate, Ag2SO4. After class: Write the letter of the best answer. 1. How many moles of H2O are in 24.0 g? a. 144.48 x 1023 b. 432.38 c. 1.41 d. 1.33 2. What is the total mass of oxygen in 146.7 g of Hg2SO4? a. 0.30 g b. 19.19 g c. 497.21 g d. 1.807 x 1023 g CHEMISTRY Chemical Compounds Unit 3. What is the percentage composition of N2O5? a. 20% N, 50% O b. 28.02% N, 80.0 % O c. 25.9% N, 74.1% O d. none of the above 4. What is the percentage of potassium in 14.8 g of KOH? a. 69.7% b. 28.5% c. 1.78% d. 56.1% 5. Solve each problem below. Show your work. a. What is the mass in grams of 9.03 moles of H2S? b. Find the percentage composition of KCl c. Calculate the percentage of nitrogen in 84.65 g of ammonium nitrate, NH4NO3. Day 17: Objectives 7.4.1, 7.4.2 Before class: Read pages 229 – 231 Answer the following. Complete sentences are not needed. 1. Define: a. empirical formula Example: A compound is analyzed and found to contain 36.70% potassium, 33.27% chlorine, and 30.03% oxygen. What is the empirical formula of the compound? You try: Determine the empirical formula of the compound that contains 17.15% carbon, 1.44% hydrogen, and 81.41% fluorine. Example: A 60.0 g sample of tetraethylead, a gasoline additive, is found to contain 38.43 g lead, 17.83 g carbon, and 3.74 g hydrogen. Find its empirical formula. You try: A 170.00 g sample of an unidentified compound contains 29.84 g sodium, 67.49 g chromium, and 7.67 g oxygen. What is its empirical formula? Discuss: Find the empirical formula of a compound that contains 53.70% iron and 46.30% sulfur. After class: Write the letter of the best answer. Which statement is true? a. The empirical formula and molecular formula for a given compound are always the same. b. The molecular formula of a substance tells how the atoms in the molecule are connected. c. The molecular formula gives the actual number and types of atoms in the molecule. d. The percent composition of a compound and the molecular weight are not sufficient information to determine the molecular formula. Solve each problem below. Show your work. What is the empirical formula of a compound that is 51.4% Cu, 38.8% O, and 9.7% C? What is the mass in grams of 0.257 mol of sucrose, C12H22O11? Calculate the percent composition of the compound C2H4O2. Day 18: Objectives 7.4.3, 7.4.4 Before class: CHEMISTRY Chemical Compounds Unit Read pages 232 – 233 Answer the following. Complete sentences are not needed. 1. Define: a. molecular formula 1. Example: The empirical formula for trichloroisocyanuric acid is OCNCl. The molar mass of this compound is 232.41 g/mol. What is its molecular formula? 2. Example: Determine the molecular formula of a compound with an empirical formula of NH2 and a formula mass of 32.06 amu. 3. You try: Determine the molecular formula of the compound with an empirical formula of CH and a formula mass of 78.110 amu. 4. Example: If 4.04 g of N combine with 11.46 g of O to produce a compound with a formula mass of 108.0 amu, what is the molecular formula of this compound? 5. You try: The molar mass of a compound is 92 g/mol. Analysis of a sample of the compound indicates that it contains 0.606 g N and 1.390 g O. Find its molecular formula. After class: 1. Solve each problem below. Show your work. a. The molar mass of NaSO2 is 174 g/mol. What is the molecular formula of the compound? b. What is the molecular formula of a substance with a molar mass of 28.0 g/mol and an empirical formula of CH2? c. What is the molecular formula of a substance with a molar mass of 216 g/mol and an empirical formula of C3H2O? d. What is the molecular formula of a substance with a molar mass of 116.07 g/mol that is 41.39% carbon, 3.47% hydrogen, and 55.14% oxygen? e. What is the molecular formula of a substance with a molar mass of 168.19 g/mol that is 64.27% carbon, 7.19% hydrogen, and 28.54% oxygen? Day 19: Objectives 7.4.2, 7.4.3 Before class: Read the lab on pages 813 – 815. We will be skipping procedure #12. Day 20: All Objectives During class: The following questions are suggested for study. They are not graded. They are not required. I recommend reading through them, and working through the ones that don’t seem obvious to you. Chapter 6 review 2, 6, 9, 15, 16, 22, 24, 27, 29 – 32, 48 Chapter 7 review 2, 4 – 7, 9 – 11, 17, 18, 20 – 23, 26, 33, 34, 36, 38, 39