Acid, base, Ph

PROPERTIES OF ACIDS
• A tart or sour taste
• Can be strong or weak electrolytes (conduct electricity)
• React with metals to produce hydrogen gas!
• Examples: Lemons contain citric acid
IS IT AN ACID?
1. Gastric Juice
2. Pure Water
3. Lemon Juice
4. Coffee
5. Sea water
6. Milk
1. Yes
2. No
3. Yes
4. Yes
5. No
6. Yes, barely
ARRHENIUS ACIDS AND BASES
• Swedish chemist in 1887
• Defined acids and base according to their ions!
ARRHENIUS ACIDS
Hydrogen containing compounds to produce H +
There are 6 (sometimes 7)strong acids
HCl, HI, HBr, HClO
3
, HClO
4
, HNO
3
,
H
2
SO
4
DID YOU KNOW?!
• A strong acid completely dissociates!
• Meaning it completely breaks down in H + ions!
• Way to go Arrhenius!
Hypothesize: would a weak acid completely dissociate?
DOES THE NUMBER OF H’S MATTER?
H
NO
3
H
2
SO
4
H
3
PO
4
H
2
S
YES!
MONOPROTIC, DIPROTIC, TRIPROTIC
M O N O P R O T I C
1 hydrogen able to ionize
D I P R O T I C
2 hydrogens able to ionize
T R I P R O T I C
3 hydrogens able to ionize
(1 reactions) (2 reactions) (3 reactions)
WHY PROTIC?
NOT HYDROGEN?
How many protons, neutrons, electrons?
Think about H + …
What’s left over?
Only a proton, so H + is commonly referred to as a proton
MONOPROTIC, DIPROTIC, OR
TRIPROTIC?
1. HClO
3
1. Monoprotic
2. Diprotic 2. H
2
SO
3
3. H
3
PO
3
4. HF
3. Triprotic
4. Monoprotic
5. HCl
6. H
2
S
7. H
3
PO
4
5. Monoprotic
6. Diprotic
7. Triprotic
BINARY ACID NOMENCLATURE
• Made of 2 different elements
• One will be hydrogen and the other will be a highly electronegative element
• Inorganic acids
Not containing carbon
BINARY ACID NOMENCLATURE
• MUST have a hydro prefix then stem of the other element and –ic
• Hydro- stem- ic and Acid
• HCl ?
• Hydro-chlor-ic Acid
BINARY ACID NOMENCLATURE
Name these:
1.
HF
2.
HCl
3.
HBr
4.
HI
OXYACIDS
• Acids with oxygen
– Typically containing H, O, and another nonmetal
– Ternary- meaning 3 elements
• Like our polyatomics
C
Hg
NH
2
H
2
+2
4
+1
3
O
2
-
CH
3
COO -
CN -
HCO
C
CO
2
O
ClO -
-2
3
3
-
4
-2
ClO
ClO
ClO
PO
PO
HPO
4
4
-
-3
2
-
3
-
-3
3
4
-2 dimercury (I) ammonium acetate acetate cyanide carbonate hydrogen carbonate oxalate hypochlorite chlorite chlorate perchlorate phosphate phosphite hydrogen phosphate
CrO
Cr
2
O
MnO
MnO
NO
NO
2
4
-
3
-
-2
4
7
-2
OH -
4
-
-2
SiO
3
-2
SCN -
S
SO
SO
2
O
3
-2
4
-2
HSO
HSO
3
4
-
3
-
-2 chromate dichromate permanganate manganate nitrite nitrate hydroxide silicate thiocyanate sulfite
Sulfate hydrogen sulfate hydrogen sulfite thiosulfate
NAMING OXYACIDS
• Whatever the charge of the polyatomic is tells you how many hydrogen’s you need
• For example: CO
3
-2 would be H
2
CO
3
• NO HYDRO PREFIX!!!!
• Change –ate to –ic and add acid
• Carbonate carbonic acid
NAMING OXYACIDS
• PO
4
-3
Phosphate
• NO
3
-1 Nitrate
NAMING OXYACIDS
• If the ending is –ite, change the ending to – ous and add acid
• For example: NO
2
is nitrite
HNO
2 or nitrous acid
NAMING OXYACIDS
To remember think of 300, leonidas (leon-ite-ous)
PO
3
-3
Phosphite
SO
2
-2 Sulfite
ACID NOMENCLATURE REVIEW
Hydro- stem- ic acid
-ate ic acid
-ite ous acid
Name or write the formula for the following acids:
1. HClO
3
2. H
2
SO
3
3. H
3
PO
4
4. Hydrofluoric Acid
5. Hydrochloric Acid
6. Phosphorous
7. Hydrosulfuric acid
1. Chloric Acid
2. Sulfurous Acid
3. Phosphoric Acid
4. HF
5. HCl
6. H
3
PO
3
7. H
2
S
PROPERTIES OF BASES
• A bitter taste
• Slippery feel like soap
• Also, form strong or weak electrolytes
• Examples: Soaps, household cleaning products
IS IT A BASE?
1. Lemon juice
2. Sea water
3. Pure water
4. Blood
5. Ammonia
1. No
2. Yes
3. No
4. Yes, barely
5. Yes
ARRHENIUS BASES
Hydroxide containing compounds to produce OH -
DECIDE WHETHER IT IS AN ARRHENIUS ACID OR
BASE?
1. Acid
1. HNO
3
2. KOH
2. Base
3. Acid
3. H
2
SO
4
4. Sr(OH)
2
5. H
3
PO
4
6. H
2
S
4. Base
5. Acid
6. Acid
BASE NOMENCLATURE
• What is the name for OH ?
HYDROXIDE
• Group 1 and 2 make strong bases (Except the 1 st two of each group and last ones)
• Metal + Hydroxide NaOH is Sodium Hydroxide
NAME THE FOLLOWING BASES:
1. KOH
2. Sr(OH)
3. Ba(OH)
4. CsOH
2
2
1. Potassium hydroxide
2. Strontium hydroxide
3. Barium hydroxide
4. Cesium hydroxide
WHAT IF A COMPOUND DOES NOT HAVE AN H + OR
OH ?
WE NEEDED TO HAVE OTHER RULES FOR FIGURING
THIS OUT
BRONSTED-LOWRY
A C I D S
A hydrogen (H + ) donor
B A S E
A hydrogen (H + ) acceptor
NH
3
+ H
2
O NH
4
+ +OH -
BASE ACID
CLASSIFY THE ACID/BASE IN THE FOLLOWING
ACCORDING TO BRONTED-LOWRY
•
HCl + H
2
O
Cl
-
+ H
3
O
+
ACID BASE
•
H
2
O + H
2
SO
4
BASE ACID
HSO
4
-
+ H
3
O
+
•
Fill in the following chart
Theorist Acid
Arrhenius
Bronsted-
Lowry
Base
WHAT ABOUT THE PRODUCTS?
• They are called conjugates, in Latin, means to join together
• With the reactants, they are known as conjugate acid-base pairs
• A pair meaning out two will be together: no more, no less
CONJUGATES
• The conjugate will be the opposite strength and type on the product side
• An acid will have a conjugate base
• A base will have a conjugate acid
• HCl + H
ACID
2
BASE
O Cl + H
3
O +
• H
2
O + H
2
SO
4
BASE ACID
HSO
4
+ H
3
O +
TRY THESE:
• HNO
3
+ H
2
O H
3
O + + NO
3
-
Acid Base Conj. Acid Conj. Base
• H
2
O + HF F + H
3
O +
Base Acid Conj. Base Conj. Acid
BUT WHAT IF THEY DON’T HAVE A PROTON (H + ) TO
DONATE!?!!?
LEWIS (LAST, BUT NOT LEAST THEORIST)
A C I D
Accepts a pair of electrons to form a covalent bond
B A S E
Donates an electron pair to form a covalent bond
H + + OH H
2
O
Acid base
LEWIS ACID OR BASE?
DRAW THE LEWIS STRUCTURE TO HELP
1. H
2
S 1. Lewis Base
2. Lewis Acid 2. BCl
3
3. PF
3
3. Lewis Base
REVIEW TOPICS FOR QUIZ
Decide the B.L Acid/base and conjugates:
1. NH
3
+ H
2
O NH
4
+ + OH -
2. HBr + H
2
O H
3
O + + Br -
3. Notice anything special about water?
AMPHOTERIC
•
Water is one of the few that can act as an
acid or a base depending on what it is mixed with
•
Other examples: NH
3
and H
2
S
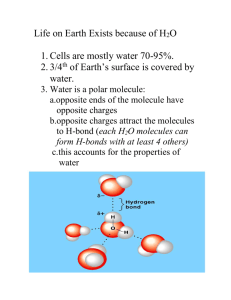
SELF IONIZATION OF WATER
•
Water is pretty special and is constantly undergoing a reaction
•
H
2
O + H
2
O
OH
-
+ H
+
•
This is happening so fast, we don’t even realize it!
SELF IONIZATION OF WATER (KW)
• Kw is the self ionization constant of water
• Kw = [H + ] x [OH ]
• The bracket [ ] mean concentration
• Kw is equal 1.00x10
-14 M 2
• For a neutral solution [H + ] = [OH ] = 1.00x10
-7 M
KW PROBLEMS
• If the [H + ] is 1.00x10
-5 M, what is the [OH ] ? Is this solution neutral, acidic, or basic?
• Step one: Figure out what you have
Kw = [H + ] x [OH ]= 1.00x10
-14 M 2
• Step two: plug in your values and solve for [OH ]
1.00x10
-14 M 2 = [1.00x10
-5 M] x [OH ]
1.00x10
-9 M
Step three: decide what range it is in
Acidic because the H + is greater than the OH -
TRY THESE ON YOUR OWN
•
Calculate the [H
+
] concentration, if the [OH
-
] is
3.0x10
-2
[H
+
]=3.3x10
-13
M
•
Calculate the [OH
-
], if the [H
+
] is 6.0x10
-10
M
ION PRODUCT CONSTANT (KW)
•
Neutral- equal amounts of [H
+
] = [OH
-
]
•
Acidic- [H
+
] > [OH
-
]
•
Basic- [H
+
] < [OH
-
]
DECIDE WHETHER ACID, BASE, OR NEUTRAL:
1. [H+]=6.0x10
-10 M
2. [OH-]=3.0x10
-2 M
3. [H+]=2.0x10
-7 M
4. [OH-] =1.0 x10 -7 M
1. Basic
2. Basic
3. Acidic
4. Neutral
• The power of hydrogen!
• Also, known as pH!
• Jot down anything you already know about pH!
THE
P
H CONCEPT
• Proposed in 1909 by Soren
Sorenson as a more practical way to express the [H+]
THE
P
H CONCEPT
• pH = -log [H+]
• If the pH is from <7 it is an acid
• If the pH is 7 it is a neutral
• If the pH is >7 it is a base
pH Common Items
pH Scale
PUTTING LOG IN OUR CALCULATOR!
LET’S TRY ONE!
• What is the pH of a solution with a [H+] of 4.2x10
-10 M?
Is it an acid or base
• > than 7, so it’s a BASE!
PST..
• pH will not have a unit
TRY THESE ON YOUR OWN
• Calculate the pH of the following and decide if an acid or base:
1.
[H + ]= 0.045 M
2.
[H + ] = 8.7x10
-6 M
3.
[H + ] = 0.0015 M
4.
[H + ]= 1.0x10
-12 M
1. 1.35; acid
2. 5.06; acid
3. 2.82; acid
4. 12; base
HELPFUL HINT HERE!
• If the coefficient (number in front) is 1, then the exponent is the pH!!
• Example: 1.00 x 10-7 M is… pH= 7.00
• What about 1.00 x 10 -9 is… pH = 9.00
Hold your applause
BUT WHAT IF YOUR GIVEN [OH-]?!?!
• Use your Kw equation!
• What is the pH of a solution with [OH-] of 3.00x10
-2 M?
•
[H
+
]=3.33x10
-13
M
• Then plug it into pH = - log (3.33x10-13 )= 12.5
• Calculate the pH for the following and decide acid or base
1. [H+]=1.00x10-6 M
2. [H+] =0.000200 M
3. [OH ] = 5.60x10
-2 M
• pH=-log[H+]
1. 6.00; acid
2. 3.7; acid
3. 12.7; base
DID YOU KNOW?!?
• Log is a base ten function!
• This comes in handy if we are given pH and have to find
[H+]!
• pH = -log[H + ] when you divide by log to solve for [H + ] it becomes the antilog or 10!
• [H + ] = 10 -pH
LET’S TRY ONE TOGETHER
• Calculate the [H+] for a solution with a pH of 5.00
• [H + ] = 10 -pH
• [H + ] = 10 -5.00 = 1.00x10
-5 M
TRY THESE ON YOUR OWN
• Calculate the [H+]
1. pH= 8.56
2. pH=3.45
3. pH= 10.57
• [H + ] = 10 -pH
1. 2.57x10
-9 M
2. 3.55x10
-4 M
3. 2.692x10
-11 M
MEASURING
P
H
• We can calculate pH, but how do you think we can measure it?
• Have you measured pH before? Maybe with a pool?
MEASURING P
H
• Using acid/base indicators
• Typically a weak acid or base and turns color at different pH’s
• Some indicators work better for certain solutions and only at 25 o C
• Examples: Brothymol blue, phenolphthalein, universal, pH paper, etc