Developmental Biology, 9e
advertisement

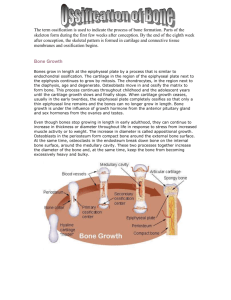
BIOL 370 – Developmental Biology Topic #14 Paraxial and Intermediate Mesoderm Lange In this chapter we shall feel further development of the mesoderm and the endoderm: • the endoderm will form the digestive and respiratory tubulature and organs • the mesoderm generates all the intermediate organs between the ectoderm and endoderm Figure 11.1 Major lineages of the amniote mesoderm (Part 1) • Intermediate mesoderm kidneys, gonads • Chordamesoderm notochord • Paraxial mesoderm head, somites • somites cartilage, tendons, skeletal muscle, dermis, endothelial cells • Lateral plate mesoderm circulatory system, body cavities Figure 11.2 Gastrulation and neurulation in the chick embryo, focusing on the mesodermal component It is important at this stage to work on developing an ability to visualize the 3-D perspective of the organism from these sections. Figure 11.4 Formation of new somites Somites are bilaterally paired blocks of mesoderm that form along the anterior-posterior axis of the developing embryo. An alternative term used in place of somites is metamere. Figure 11.4 Formation of new somites (Part 2) New somites are formed in the process of somatogenesis, which is both molecular and cellular in origin. Of key interest in the somite formation below is the use of ephrins (also known as ephrin ligands (abbreviated Eph)). These are a family of proteins that serve as the ligands of the ephrin receptor.. Figure 11.5 Notch signaling and somite formation (Part 4) the Notch ligand is produced by the Delta-like 3 gene. “E” shows a Delta-like 3 gene knockout mouse with a clearly aberrant skeleton. White dots show the pattern of ossification centers in both mice. Figure 11.7 Hypothetical pathway for regulation of the clock through which an Fgf8 gradient regulates a Wnt oscillating clock The pathway for control of the genes regulating somite formation are shown below. One of the most pressing issues we have poor understanding of currently is how the timing of these events is regulated and coordinated. Figure 11.10 When segmental plate mesoderm is transplanted it differentiates according to its original position In this chronologically backwards transplant study, older donor mesodermal tissue is transplanted to an earlier stage embryo in a different location. The resultant donor structure is already fixed and it continues development into its original presumptive structures… in this case, into vertebrae now developing in the cervical neck region. Transverse section through the trunk of a chick embryo on days 2–4 Somites visible as the red structures are multipotent at this point and their specification is dependent upon their location relative to paracrine factors received from surrounding tissues (such as the neural tube, epidermis, etc.) Figure 11.11 Transverse section through the trunk of a chick embryo on days 2–4 (Part 1) Dermamyotome – the segment of the somite that is going to form the dermis (dermatome) and the skeletal muscle (myotome). The combined name is used because the initial development is slower than in other somite segments. Figure 11.11 Transverse section through the trunk of a chick embryo on days 2–4 (Part 2) Notice now the separate dermatome and myotome. Figure 11.12 Primaxial and abaxial domains of vertebrate mesoderm (Part 1) Primaxial simply refers to the portion of the mesoderm that is more “medial” and the abaxial is more distal to the center axis. Remember from earlier discussions the term “epiblast” which is referring to the upper layer. Figure 11.14 Ablating Noggin-secreting epiblast cells results in severe muscle defects Epiblastic mesodermal cells that are experimentally ablated (destroyed, usually chemolytically or electrolytically) result in severe muscle defects arising in the chick. Figure 11.15 Conversion of myoblasts into muscles in culture Notice the different paracrine factors that will facilitate the different steps. FGF = fibroblast growth factor CAM = cell adhesion molecule Figure 11.16 Schematic diagram of endochondral ossification Chondrocytes – cartilage producing cells Osteocytes - cells within the calcified aspect of bone Osteoblasts - cells that synthesize bone Osteoclasts – cells that degrade bone. Figure 6.9 Endochondral ossification in a long bone. Month 3 Week 9 Birth Childhood to adolescence Articular cartilage Secondary ossification center Epiphyseal blood vessel Area of deteriorating cartilage matrix Hyaline cartilage Spongy bone formation Bone collar Primary ossification center 1 Bone collar Spongy bone Epiphyseal plate cartilage Medullary cavity Blood vessel of periosteal bud 2 Cartilage in the 3 The periosteal center of the forms around hyaline cartilage diaphysis calcifies and then develops model. cavities. bud invades the internal cavities and spongy bone begins to form. 4 The diaphysis elongates and a medullary cavity forms as ossification continues. Secondary ossification centers appear in the epiphyses in preparation for stage 5. 5 The epiphyses ossify. When completed, hyaline cartilage remains only in the epiphyseal plates and articular cartilages. Figure 6.11 Long bone growth and remodeling during youth. Bone growth Cartilage grows here. Bone remodeling Articular cartilage Epiphyseal plate Cartilage is replaced by bone here. Cartilage grows here. Cartilage is replaced by bone here. Bone is resorbed here. Bone is added by appositional growth here. Bone is resorbed here. Steel “Bone Cages” used to lengthen legs. These were originally developed in the Soviet Union in the 1950s to treat dwarfism. An example of untreated acromegaly. Figure 11.17 Endochondral ossification In this image we see a potential pathway for the transition of cartilage into bone…. The formation of endochonral ossification. Figure 6.17 Fetal primary ossification centers at 12 weeks. Parietal bone Occipital bone Mandible Frontal bone of skull Clavicle Scapula Radius Ulna Ribs Humerus Vertebra Ilium Tibia Femur Figure 11.18 Skeletal mineralization in 19-day chick embryos that developed (A) in shell-less culture and (B) inside an egg during normal incubation The shell of the egg is the primary source of calcium for ossification of the bird skeleton prior to hatching. Figure 11.21 Scleraxis is expressed in the progenitors of the tendons • Scleraxis expressing progenitor cells lead to the eventual formation of tendon tissue and other muscle attachments. • Scleraxis is also associated with embryonic tissues that develop into tendon and blood vessels. Aortic dissection is a disorder that is often due to an abnormality in scleraxis of the aorta. The late John Ritter is one person to have died due to this condition. Figure 11.22 Induction of scleraxis in the chick sclerotome by Fgf8 from the myotome Scleraxis expressing progenitor cells lead to the eventual formation of tendon tissue and other muscle attachments.[ Figure 11.23 General scheme of development in the vertebrate kidney Pronephros - the most basic of the excretory organ that develops in vertebrates Mesonephric tubules - form to attach to the mesonephros as the pronephros degenerate Metanephros – the final mammalian kidney Figure 11.24 Signals from the paraxial mesoderm induce pronephros formation in the intermediate mesoderm of the chick embryo Figure 25.3b Figure 25.4b Figure 11.25 Reciprocal induction in the development of the mammalian kidney Figure 11.26 Kidney induction observed in vitro Figure 11.32 Development of the bladder and its connection to the kidney via the ureter End.