Balancing Redox Equations and Voltaic Cells
advertisement
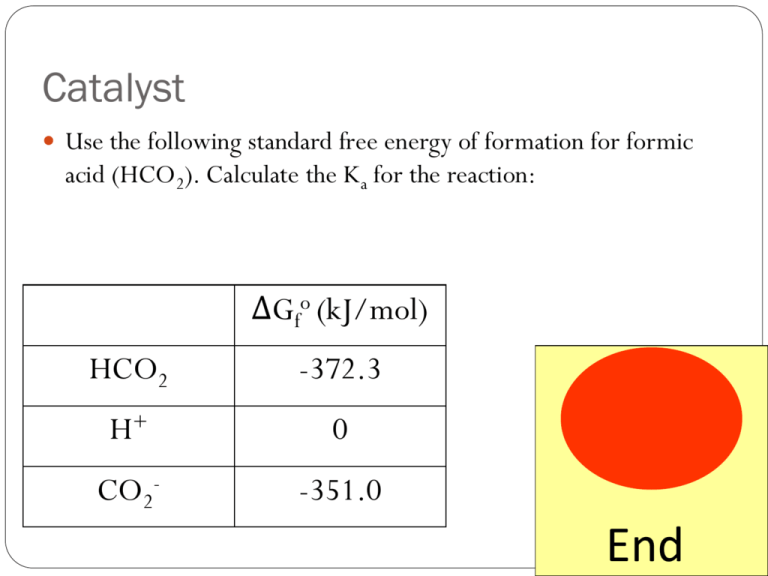
Catalyst Use the following standard free energy of formation for formic acid (HCO2). Calculate the Ka for the reaction: ΔGfo (kJ/mol) HCO2 -372.3 H+ 0 CO2- -351.0 End Lecture 8.4 – Balancing Redox Reaction and Galvonic Cells Today’s Learning Targets LT 8.9 – I can identify the compounds that are being oxidized and reduced in a chemical reaction. LT 8.10 – I can balance a redox reaction in acidic and basic solutions by utilizing the half reactions for a given oxidation-reduction reaction. LT 8.11 – I can draw a voltaic (galvanic) electrochemical cell for a given redox reaction. In this drawing, I can identify, the anode, cathode, salt bridge, direction of election movement, and placement of oxidation and reduction half reactions. LT 8.12 – I can calculate the standard reduction potential for an electrochemical cell through utilization of a reduction potential table. LT 8.13 – I can identify an oxidizing agents and reducing agents in a chemical reaction and utilizing a reduction potential table. Oxidation – Reduction Reactions Oxidation-Reduction reactions (or redox reactions) are reactions that involve the transfer of electrons from one element to another element A substance either gives or receives electrons LEO the lion says GER Lose Electrons Oxidized Gain Electrons Reduced Assigning Oxidation Numbers In order to identify substances that are gaining or losing electrons, we assign oxidation numbers. 1. An atoms in element form have a state of 0 2. A monoatomic ions oxidation number equals its charge (e.g. K+ = +1 oxidation number). This also applies to monoatomic ionic compounds. 3. Non-metals have the charge of their group usually: Oxygen is always -2 except in peroxides (O22-) where it is -1 Hydrogen is usually +1 when bonded to nonmetals and -1 when bonded to metals Halogens almost always are -1. Fluorine is always -1, all other halogens may have a positive charge 4. The sum of the oxidation numbers in a compound equal the charge of the compound. Class Example Assign the oxidation states to the following elements/compounds and identify the substances being oxidized and reduced: 2 Al (s) + 6 HBr (aq) 2 AlBr3 (aq) + 3 H2 (g) Table Talk Assign the oxidation states to the following elements/compounds and identify the substances being oxidized and reduced: Mg (s) + CoSO4 (aq) MgSO4 (aq) + Co (s) Steps to Balancing Redox Reactions Divide each reaction into two ½ reactions 2. Balance each ½ reaction 1. First balance elements other than H and O 2. Balance O by adding H2O as needed 3. Balance H by adding H+ as needed 4. Finally, balance chare by adding e- as needed 3. Multiply each half reaction so that there are equal number of electrons in each half reaction 4. Sum the reactions together. 1. Class Example Balance the following reaction for both mass and charge in acidic solution: MnO4- (aq) + C2O42- (aq) Mn2+(aq) + CO2 (aq) Table Talk Balance the following reaction for both mass and charge in acidic solutions: Cr2O72- (aq) + Cl- (aq) Cr3+ (aq) + Cl2 (g) Table Talk Balance the following reaction for both mass and charge in acidic solutions: Cu (s) + NO3- (aq) Cu2+ (aq) + NO2 (g) Balancing in Basic Solution By adding hydrogen to each side, we create an acidic solution If the redox reaction occurs in basic solution, add equal amounts of OH- on each side. The OH- reacts with the H+ to make water Class Example Balance the following reaction for both mass and charge in basic solution: CN- (aq) + MnO4- (aq) CNO- (aq) + MnO2 (s) Table Talk Balance the following reaction for both mass and charge in basic solutions: NO2- (aq) + Al (s) NH3 (aq) + Al(OH)4- (aq) Table Talk Balance the following reaction for both mass and charge in basic solutions: Cr(OH)3 (s) + ClO- (aq) CrO42- (aq) + Cl2 (g) Catalyst Balance the following reaction for both mass and charge in acidic solutions: Cr2O72- (aq) + Cl- (aq) Cr3+ (aq) + Cl2 (g) End Fill the Box! There are nine questions around the room Balance the reactions and fill in the handout as you complete the activity. Voltaic Cells The energy released in a spontaneous redox reaction can be used to perform electrical work. This is done through the construction of a voltaic cell In a voltaic cell, the two half reactions are split and they are not in direct contact with one another The movement of electrons between the two cells can be utilized to do work Important Parts of Voltaic Cells Anode – The site where oxidation takes place. Negatively charged. Cathode – The site where reduction takes place. Positively charged. Salt Bridge – Connects the two ½ cells and contains an unreactive salt. This ensures that charge does not build up in the ½ cells Common salts solutions are NaNO3 or KNO3 Class Example The oxidation – reduction reaction: Cu2+ (aq) + Zn (s) Zn2+ (aq) + Cu (s) Draw the setup for the voltaic cell that can be created in order to do work. Assume KNO3 is utilized for the salt bridge. Identify, the anode, cathode, salt bridge, the charge of the two electrodes, movement of elections, and write the voltaic cell in cell notation. Table Talk The oxidation – reduction reaction: ClO3- (aq) + 6 H+ (aq) + 3 Zn (s) 3Zn2+ (aq) + Cl- (aq) + 3 H2O (l) Draw the setup for the voltaic cell that can be created in order to do work. Assume KNO3 is utilized for the salt bridge. Identify, the anode, cathode, salt bridge, the charge of the two electrodes, movement of elections, and write the voltaic cell in cell notation. Cell Potentials In a voltaic cell, electrons move from an area of high potential energy to an area of low potential energy Differences in potential energy are measured in volts J 1V = 1 C The potential difference between two cells is the cell potential (Ecell) When the cell is run under standard conditions, it is called the cell potential (Eocell) standard Standard Reduction Potentials We measure numerous half cells under standard conditions (25 oC, 1 M, and 1 atm) We can calculate the standard reduction potential (Eocell) for the electrochemical cell by utilizing: o o E ocell E cathode E anode Therefore, we can calculate the electrochemical potential for a voltaic cell simply by using a reference table Insert Table 20.1 Class Example A voltaic cell is set up for the following reaction: Zn (s) + Cu2+ (aq, 1 M) Cu (s) + Zn2+ (aq, 1 M) Using Table 1, calculate the Eocell for this voltaic cell Table Talk You set up the following voltaic cell: Cr2O72- (aq) + 14 H+ (aq) + 6 I- (aq) Cr3+ (aq) + 3 I2 (s) + 7 H2O (l) Calculate the Eocell for the voltaic cell Oxidizing and Reducing Agents The substance that causes another object to be oxidized is known as the oxidizing agent (it itself it reduced) The substance that causes another object to be reduced is known as the reducing agent (it itself it oxidized) The higher you are on the reduction potential table, the better an oxidizing agent you are The lower you are, the better a reducing agent you are when the reaction is reversed Better reducing agent! Better oxidizing agent! Insert Table 20.1 Class Example Using your reduction potential table, rank the following ions in order of increasing strength as oxidizing agents: NO3-, Ag+, Cr2O72- Table Talk Using your reduction potential table, rank the following ions in order of increasing strength as reducing agents: I-, Fe, Al White Board Problems White Board Problems You create a voltaic cell with the following reaction: Fe (s) + 2 Ag+ (aq) Fe2+ (aq) + 2 Ag (s) Create a diagram of the two half cells. Label the cathode, anode, movement of electrons, salt bridge, and write in cell notation. Calculate the standard reduction potential for the reaction: Cl2 + 2 I- (aq) 2 Cl- (aq) + I2 (s) Calculate the standard reduction potential for the reaction: Ni (s) + 2 Ce4+ (aq) Ni2+ (aq) + 2 Ce3+ Calculate the standard reduction potential for the reaction: Cu2+ (aq) + Ca (s) Cu (s) + Ca2+ (aq) Which of the following is a stronger oxidizing agent: Fe (s) or Mg (s) Which of the following is a stronger reducing agent: Cl2 (g) or Br2 (l) Closing Time Read 20.2, 20.3, and 20.4 Homework: 20.6, 20.8, 20.22, 20.23, 20.27, 20.37, 20.38, 20.39, and 20.43 Quiz Thursday/Friday Saturday School this weekend!