CP Chemistry Unit 8: Redox and Electrochemistry *The small test
advertisement

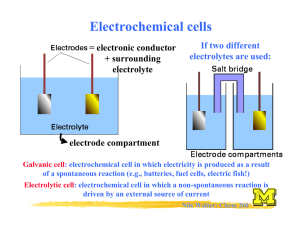
CP Chemistry Unit 8: Redox and Electrochemistry *The small test/large quiz will be free response (~25-30 points, 45 minutes). *Review dates: In-class review time, Lunch 5/23, and 7th period 5/27. *Bring a SCIENTIFIC CALCULATOR to the test (like usual). *You will get a periodic table, polyatomic ion sheet, and standard reduction potential table for the test. **Answer key is posted on my website! Suggestion: I would treat this like a “practice test” since you have already had ample practice with the in-class worksheet. Obviously, this practice is much longer than what you would actually get for the test but these are the types of questions you should expect to see. Concepts: Oxidation numbers Oxidation Reduction Redox reactions Half-reactions o Balancing reactions using the halfreaction method Electrochemistry Voltaic cell o Half-cell o Electrode o Anode o Cathode o Salt bridge Cell notation Reduction potential Cell potential Practice Questions: 1. What is a redox reaction? What are transferred in a redox reaction? 2. In a redox reaction, how do you know which species has been oxidized or reduced? 3. What are half-reactions? 4. What is a voltaic cell? How does it work? 5. What is the function of the salt bridge in a voltaic cell? 6. What is reduction potential? 7. What reaction happens at the cathode? At the anode? 8. What does it mean if the cell potential is negative? Practice Problems: (solutions posted on my website) 1. Assign oxidation numbers: a. NF3 e. N2H4 i. (NH4)2(CrO4) b. Ba(NO3)2 f. MnO2 j. H3O+ c. ClO4- g. MgCO3 k. CN- d. N2O h. CO 2. Identify the half-reactions and balance the following reactions (*remember, after you balance the electrons in the half-reactions transfer the coefficients to the original equation. Don’t change these anymore! If oxygen needs to be balanced, use H2O. If hydrogen needs to be balanced, use H+): a. CH4 + O2 CO2 + H2O b. Ag + NO3- + H+ Ag+ + NO + H2O c. NaBr + Cl2 NaCl + Br2 d. Al + H+ + Cl- Al3+ + H2 + 2Cl- e. Cu + SO42- + H+ Cu2+ + SO2 + H2O 3. Fe2+ + 2e- Fe Cu2+ + 2e- Cu a. Write the overall cell reaction for the given pair of half-reactions. b. Write the cell notation. c. Calculate E0cell. 4. Rb+ + e- Rb Au3+ + 3e- Au a. Write the overall cell reaction for the given pair of half-reactions. b. Write the cell notation. c. Calculate E0cell. 5. Zn2+ + 2e- Zn Ag+ + e- Ag a. Write the overall cell reaction for the given pair of half-reactions. b. Write the cell notation. c. Calculate E0cell. d. Sketch the voltaic cell. Be sure to label: Electrodes Solution Direction of electron flow Anode Cathode Salt bridge (draw where ions go!)