Chapter 3 - The Chemistry of Organic Compounds
advertisement

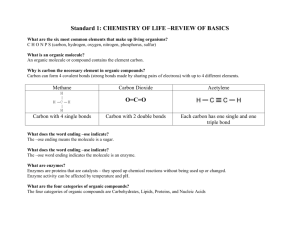
The Chemistry of Organic Molecules Scientists used to believe cells contained something “extra” that other matter did not; a vital force of some kind. They divided chemistry up into “living” (organic) and “nonliving” (inorganic) chemistry. “organic molecules” were thought to relate specifically to life processes. We now know that the chemical reactions of life are just like any other chemical reaction. The most abundant “life-specific” materials, however, are carbon and hydrogen. Therefore: “Organic molecules” are molecules containing carbon and hydrogen. Inorganic Molecules do not. Matter Is made of… Elements That combine to form… Molecules & Compounds Made from a carbon backbone and … Which Include… Organic Compounds Nucleic Acids Functional Groups Lipids hydroxyl amino carbonyl phosphate Proteins carboxyl Sugars sulfhydryl Which, together with backbone structure lead to specific… Hydrophobic Chemical Properties Hydrophilic Acidic Alkaline Polar Nonpolar Organic Compounds: carbon + hydrogen ALL complex organic molecules are built from the same basic carbon scaffold idea. What makes them different? – Carbon skeleton’s structure – Functional Groups Cells contain 4 major classes of organic compounds: – Proteins (made of amino acids) – Carbohydrates (sugars) – Lipids (fats, oils, etc.) – Nucleic Acids (DNA, RNA) We name organic compounds according to their functional groups and chemical properties. Because of the carbon atom’s unique structure, it is capable of forming a great diversity of different structures. It is this property of carbon that contributes to life’s rich diversity on all levels of the biosphere Key concept: FORM FOLLOWS FUNCTION Show inner life of the cell A chemical anagram Isomers share the same chemical formula – they are composed of the same atoms – but have different architecture. Form follows function… different shapes are going to have different functional implications. Andrew Ippolito Warden, I Lit Poop Isomers of C3H8O 1-Propanol n-propyl alcohol H H H H C C C O H H H H Both compounds have the same number of C, H, and O atoms; however, they are arranged differently. 2-Propanol isopropyl alcohol H H OH H C C C H H HH Why do isomers have different names? We name chemical compounds by how their atoms are arranged, Because different arrangements have different chemical effects. If chemical properties were not affected by the atomic arrangement, we wouldn’t care about how they were arranged – and so we wouldn’t have different names. The chemical and physical “rules” that molecules follow due to their unique nature. Chemical Properties we will be concerning ourselves with include: Solubility (how soluble the molecule is in water) Alkalinity (measure of how acidic/basic a molecule is) Reactivity (how likely a molecule is to undergo a chemical reaction) Structure (is it long and bendy, clumpy and gooey, or flat and stiff) Keep in mind why everything revolves around water. The ability of a molecule or compound to dissolve in water. – Hydrophobic: (phobia) – the molecule is afraid of water; it does not dissolve. – Hydrophilic: (phila, love) – the molecule loves water, and dissolves in it. Solubility is influenced by the functional groups present in an organic molecule Molecules are soluble in water if they are polar (they are imbued with an electrical charge – not neutral) – Examples: Basic concept (no pun intended): pH is really a measure of how chemically “reactive” the environment is. – This reactivity depends on two factors The hydrogen ion concentration (H+) The hydroxyl ion concentration (OH-) – Why? These are the breakdown products of water. Substances that contribute to an increase in the H+ concentration (or a decrease in OH- concentration!) are considered acidic. – Example: add HCl to water and the H+ concentration shoots up. Substances that contribute to an increase in OHconcentration (or a decrease in H+ concentration!) are considered basic (or “alkaline”) The reactivity of an organic molecule depends upon the electron configuration of it’s functional groups. Nearly all organic compounds react with other compounds in one way or another. Hydration, an example chemical reaction – Functional groups are parts of a molecule. – They have chemical properties that are independent on what the Rest of the molecule is made out of. An arm is an arm, whether it’s on your shoulder or a robotic car. It still grasps, it still has finger-like appendages. – They are not “functional groups” until they are actually attached to a molecule. If they are free, they are ions! (wanderers, remember?) “hydroxyl ion” “hydrogen ion”, “phosphate ion”, etc. – Functional Groups contribute to the name of a molecule (ethane vs. ethanol) – The functional groups of a molecule influence the molecules chemical properties. Example: [Rest of molecule]—OH – “OH” is the hydroxyl functional group. When a hydroxyl group is present in a molecule it increases the negative charge of a molecule (and therefore increases its polarity) – When a single hydrogen in Ethane (C2H6) is replaced by a hydroxyl group, the molecule ceases to be ethane and becomes Ethanol (C2H3O). – Ethane is insoluble in water because it is hydrophobic, but ethanol, because of its polar hydroxyl group, is hydrophillic. – An abbreviation for ethanol in chemical “shorthand” is EtOH. Carbohydrates Proteins Lipids Nucleic Acids monomer polymer Monomeric compounds Polymeric compounds Macromolecules can be thought of as chains of smaller molecules. – Each link in the chain is called a monomer. – The chain itself is called a polymer. Organic macromolecules found in all living cells are classified into 4 groups: – – – – Proteins Nucleic Acids Lipids Carbohydrates Sugars – Can be monomeric (monosaccharide) or polymeric (polysaccharide). Lipids – Any organic, fat-soluble (hydrophobic) molecule. Highly diverse family of molecules broken down into further classifications. Proteins – One or more polypeptide chains composed of amino acids, folding into complex structures Nucleic Acids – Chains of nucleotides or deoxynucleotides. Two types: simple + complex Simple: – A carbon backbone with a ratio of C:H:O of 1:2:1 (usually, not always). – simple sugars are called monosaccharides, and are universally used as immediate energy source to cells. – two simple sugars can link together by dehydration reactions to produce disaccharides C6H12O6 (generic hexose) is the simplest sugar (monosaccharide) we will deal with right now. – This compound has 3 different, common structures of its carbon backbone (isomers). – these are GLUCOSE, FRUCTOSE, AND GALACTOSE. – Different pairs of these produce the variety of disaccharides: Glu + Glu = Maltose (used in brewing) Glu + Fru = Sucrose (table sugar, made in plants) Glu + Gal = Lactose (milk) plants transport sugars in the form of disaccharides The common isomers of Glucose (6-carbon monosaccharide) link Polysaccharides are long chains of monosaccharides. POLYSACCHARIDES are used for two things: – storing energy in the short-term (they don't freely enter a cell membrane), and – structural components (crab shells) Energy Storage Polysaccharides: – Starch = Plants – Glygoen = Animals – Both are polymers of glucose; not fructose or galactose. Starch can either be branched or unbranched in plants. In animals, it is always branched. – Unbranched starch (only in plants) is called amylose. – Branched starch (in plants) is called amylopectin. Glycogen is HIGHLY branched compared to amylopectin. The shape of these polysaccharides is helical. This allows the connecting bonds to stick out in the environment, where enyzmes have access to break them. Glycogen's release depends on hormones. insulin from the pancreas promotes the storage of glucose as glycogen. Figure 3.8aa Figure 3.8ba Lipids: a diverse class of organic compounds that all share the same general chemical property: they are insoluble in water. – Lipids are made from 2 subcomponents: Glycerol (3 carbons with 3 OH groups; an alcohol) Fatty acid (a hydrocarbon chain with a carboxyl group at the very end) Lipids store more energy than sugars per unit mass. They are more “energy dense” CH bonds (predominant bonds in lipids) are stronger than CO bonds (predominant bonds in sugars) – Why? Oxygen’s hogging the electrons, so the bond is weak. This is why sugars are used as “fast energy” molecules – the bonds are much easier to break than lipids! Why are saturated fats worse for you than Unsaturated fats? – Circulatory disorders. Solids at high temperatures clog your arteries Website: The Lipid Library Triglyceride: A compound consisting of 1 glycerol attached to 3 fatty acids Triglycerides are the subunits of fats and oils. – Fats have triglycerides containing fully saturated fatty acids. – Oils have triglycerides containing unsaturated fatty acids – those containing double carbon bonds at various places along the chain. Fats are solid because their fatty acids are saturated with hydrogens, making the hydrocarbon chains linear. – Linear chains stack on each other in a highly ordered way Oils are liquid because their fatty acids are unsaturated, meaning that some carbons in the hydrocarbon chain are double bonded to each other – not hydrogen. This bond creates a kink in the chain. – Kinks allow a greater amount of movement in the chain, and are therefore less ordered, keeping them in a looser, liquid configuration Solid fats in your blood vessels inhibit blood flow. Triglyceride Glycerol C—O—C Fatty Acid C—O—C Fatty Acid C—O—C Fatty Acid Fat Glycerol C—O—C Saturated C—O—C Saturated C—O—C Saturated Oils Glycerol C—O—C Un C—O—C Un C—O—C Un Lipid droplets are the lipid storage organelles of all organisms. Their important role in cellular and organismic energy storage becomes most prominent in cases where lipid droplet biology is misregulated. – This is for example the case in several major lipid storage diseases such as atherosclerosis, diabetes or obesity. For a long time it was thought that lipid droplets only act as storage depots. More recent data, however, support the idea that lipid droplets are highly dynamic organelles which participate in several cellular processes and interact with various other cellular compartments. Despite their multifariousness of functions, all lipid droplets share a simple, stereotyped structure of a hydrophobic core built of the storage lipids (mainly triacylglycerols), surrounded by a phospholipid monolayer to which numerous proteins are attached (Fig. 1). Although the central role of lipid droplets for energy storage was demonstrated, little is known about their cellular biology such as biogenesis, mechanism of protein association or size and number control inside cells. Phospholipids – Heads that dissolve in water and tails that don’t. If left to their own devices in an aqueous solution, they will move around and align such that the heads are all facing the same way, and the tails are all facing the other way. ++++++++++++++++++++++++++++++++++++ ---------------------------------------------------------------P P P P P P P ++++++++++++++++++++++++++++++++++++ ---------------------------------------------------------------P P P P P P P The Plasma Membrane a.k.a. The Lipid Bilayer Outside of the cell = Extracellular Environment Outer Leaflet Intermembrane Space Inner Leaflet Inside the Cell: Cytoplasm THE SAME IS TRUE FOR MANY OTHER MEMBRANES Nucleus, Endoplasmic Reticulum, Golgi Apparatus, etc. Phospholipids are constructed the same way as triglycerides, except a single phosphate functional group replaces a fatty acid. Phospholipid O C—O—P C—O—C O R CCCCC C—O—C CCCCC C—O—C C—O—C Glycerol Glycerol O CCCCCC O C—O—P O R Saturated CCCCCCC C—O—C Saturated CCCCCCC O Characterized by their structure: 4 Fused Carbon rings What makes steroids different from each other? The function groups attached to the carbon rings. Basic structure of many steroids Beethoven Pneumonic: SIX SIX SIX FIIIIVE A basic steroid that serves as both a structural component and a precursor for more complex steroids. When inserted into a plasma membrane, cholesterol increases a plasma membrane’s rigidity. – Think of a stabilizing fin in a boat. Diets high in both cholesterol and fats lead to a build up of solid material in the blood vessels, leading to all sorts of trouble! Membrane in a gel state with straightened chains. The green chains are hydrocarbons, the blue elements are cholesterol, and the red spheres are the head-group atoms of the lipids. The cholesterol is removed to show the lipid chains more clearly in the visualization on the right. Membrane in a fluid state. Unlike the membrane in a gel state, the membrane in a fluid state has a low cholesterol concentration (one cholesterol molecule to 16 lipid molecules), and the hydrocarbon chains appear highly disordered. The cholesterol is removed in the visualization on the right. Website Cholestrol is also precursor to: – Testosterone = Testes; Estrogen = Ovaries Primarily – not exclusively! There are other places these are formed… Figure 3.13a Whereas a triglyceride is a small alcohol (glycerol) attached to 3 fatty acids, waxes are a long alcohol attached to a very long fatty acid. Waxes = Long fatty acids + long alcohols Cuticle in plants decreases water loss Skin and fur maintenance. – Earwax has cerumin, which repels or kills insects! – Traps dust & dirt before it reaches the eardrum Bees make a honeycomb to store the breakdown products of sucrose – Remember sucrose is a disaccharide of glucose and fructuose Proteios, “first place” – the most important organic molecule in the cell Also the most diverse As much as 50% of the dry weight of cells is protein Over 100,000 identified so far Support Enzymes Transport Defense Hormones Motion Points of confusion: – – – – Amino acid Peptide Polypeptide Protein Additional reading Amino Acids are the building blocks of proteins. Peptides are two or more amino acids joined together by a peptide bond. Polypeptides are chains of peptides. Peptide Bond Dynamics (Draw it) Dehydration Hydrolysis H2O The stupid picture in the book Phospho-acceptors (can be phosphorylated) – Serine – Threonine – Tyrosine Highly charged – Glutamic Acid (acidic) – Aspartic Acid (acidic) – Histidine (basic) Structurally Important – Alanine – Glycine – Proline (3.16 has mistake) Primary structure (“what is the protein’s sequence?”) – The sequence of amino acids in the polypeptide chain Secondary (the “local” structure of a portion of the polypeptide chain) – Stretches of amino acids will tend to form repeating units such as helices, sheets, fingers, etc. Alpha helix Beta pleated-sheet Zinc finger – Reinforced by hydrogen bonding Tertiary – The global shape of the entire polypeptide chain. Usually classified as “globular” and “filamentous”. – Globular polypeptides have hydrophobic centers for the same reason that phospholipids form a bilayer; it’s the net sum of all forces on the molecule that causes them to spontaneously settle into a stable structure. In this case, the polar areas of the chain will react with the surrounding aqueous environment, while the hydrophobic regions will aggregate towards the inside. Thus, “hydrophobic interactions” are truly a misnomer. – Reinforced inter-chain crosslinks (disulfide bonds) Quaternary – When multiple polypeptide chains (that have their own tertiary structure) come together and interact in a stable complex, they will exhibit a higher order structure called Quaternary structure. – Short version: The overall shape of 2 or more polypeptide chains interacting with each other. A protein consisting of a single polypeptide chain is said to be a monomeric protein (or simply (“monomer”) Proteins with two polypeptide chains are dimeric proteins (“dimer”) – Each polypeptide in a dimeric protein is generally refered to as a subunit There are trimeric (trimer), quatrameric (quatramer), etc… proteins. Proteins with many subunits are called multimeric proteins (multimer) – DNA and RNA polymerase complexes, nuclear pores, ion channels, and many gene regulatory proteins are multimeric protein complexes. Primary: Sequence of amino acids Secondary: folding of localized regions sheet helix Tertiary: folding of the entire polypeptide Quaternary: interaction of 2 or more polypeptides Denaturation: When a polypeptide’s tertiary struture collapses (melts) Aggregation: when a polypeptide does not fold properly, but jumbles into a mess. Watch video? Not all proteins can form their proper shapes spontaneously. Many need help as they are being synthesized. Proteins that help other proteins fold correctly are called protein chaperones. Proteins that incorrectly fold can form huge, clumpy messes called protein aggregates. Polymers of nucleotides Nucleotides are compounds composed of three modules: – Phosphate phosphoric acid – A pentose sugar sugar with 5 carbons – A nitrogenous base A nitrogen-containing molecule having the chemical properties of a base phosphate Nitrogenous Base (deoxy)Ribose NUCLEOTIDE phosphate Nitrogenous Base (deoxy)Ribose PO4 Phosphate provides high-energy chemical bonds. phosphate Nitrogenous Base (deoxy)Ribose Ribose is simply a 5-carbon sugar (a pentose). When you remove a hydroxyl functional group from the 2’ carbon, it becomes deoxyribose. (very weak bond) (very strong bond) phosphate Nitrogenous Base (RNA only) (deoxy)Ribose The 3rd component, the base, is either a purine or pyramidine. adenine Uracil Thymine guanine cytosine Purines are bigger than U AG adenine guanine Thymine cytosine Uracil pyramidines T C Nucleotides are found as – polymers DNA and RNA, which serve as information carriers – monomers as coenzymes and energy carriers (ATP) Coenzymes are essential factors that ensure enzymes can carry out their biochemical functions Adenosine Tri-Phosphate (ATP). Stores energy in chemical bonds that can be extracted by the cell to perform work. A pairs with T G pairs with C So, one big always binds with one small A and T form 2 hydrogen bonds G and C form 3 hydrogen bonds – GC is therefore harder to pull apart than AT A G A G A G A A T C Why does A always pair with T, and G always pair with C? T C T C T T So the helix is of uniform thickness Benzopyrene, the major mutagen in tobacco smoke, in an adduct to DNA.[52] Deoxyribonucleic acid (DNA) – Very stable molecule. – Primary function is to store information, such as the primary sequence of proteins Ribonucleic acid (RNA) – Not as stable as DNA – RNA does many things. Different classes of RNA are specialized to their own tasks. The two most characterized are: Messenger RNA (mRNA) encodes a protein sequence There are transfer RNAs (tRNA) specific for each of the 20 amino acids rRNA (ribosomal RNA) is an essential component of Ribosomes, which help translate the language of Genes to the language of Proteins. – Each kind of RNA has its own kind of genes. The genes we normally talk about are actually genes that encode mRNA.