a Gas
advertisement

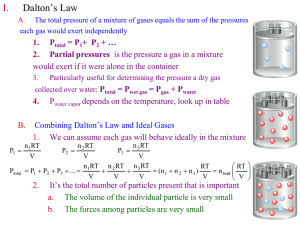
Gases States of Matter A Solid - occupies a definite volume and has a definite shape.. -atoms are in close contact A Liquid - has a specific volume but it assumes the shape of its container. - it has a ability to flow -the distances between atoms are greater than in a solid a Gas - has neither a shape of its own nor fixed volume. It takes the shape and volume of its container. - Gas mixtures are always homogeneous - Gases are highly compressible. - The molecules of a gas are relatively far away each other. - Individual gas molecules have little interaction with their neighbors. - Gas molecules move freely in every dimension of space randomly Number of gas substances are few, Pressure is a force per unit area. For gas, P= F ------A Force = (mass*acceleration) or F=ma F orce is expressed in Newtons (N) and area is expressed in square meters (m2) The SI unit of pressure is N/m2 is called Pascal (Pa) For liquid P = g ·h ·d g = gravitational force 9.81 m/s2 h = height of a column d = density of a liquid Atmospheric pressure is measured by a mercury barometer. At sea level, The standard atmospheric pressure is the pressure sufficient to support a column of mercury 760mm in height. 1.0 atm = 760 mmHg = 760 torr = 1.01325 bar = 1.01325x105 Pa In the laboratories, the gas pressures is measured by manometer. question : what is the height of a column of water that exerts the same pressure as acolumn of mercury 76.00cm high? Density of mercury is 13.6g/ml question: a) convert to 0.357 atm to bar. b) convert 147200 Pa to torr Gas laws Amount of gas, volume of gas, temperature and pressure are the fundamentals properties to determine the physical behavior of a gas. Boyle’s law (the pressure-volume relationship) Charles’s Law The temperature - volume relationship For a fixed amount of gas at a constant temperature, the gas Volume is inversely proportional to the gas Pressure. The Volume of a fixed amount of a gas at a constant pressure is directly proportional to its absolute (Kelvin) Temperature. •Boyle 1662 Pinitial . Vinitial = constant = Pfinal . Vfinal Charles 1787 Gay-Lussac 1802 Vinitial Vfinal ----------- = ---------Tinitial Tfinal In 1848 William Thomson (Lord Kelvin) proposed an absolute temperature scale known as Kelvin scale. On this scale 0°K is called absolute zero, equals -273.15°C. T (K) = t ( C) +273.15 the standard temperature for gases is defined as 0 C=273.15K and the standard pressure is defined as 1atm=760mmHg. These Standard conditions are usually abbreviated as STP. P = 1 atm = 760 mm Hg T = 0°C = 273.15 K At 1.0 atmosphere pressure and 0°C, 1 mole of any gas (i.e. 6.02 x10over23 gas molecules) occupies approximately 22.4 liters volume. At STP 1 mol gas = 22.4 L gas If the proportionality constant is called "R", This equation is known as the ideal-gas equation . An ideal gas is a gas whose physical behavior is accurately described by the ideal-gas equation. Values for the gas constant R Units Value 0.08206 Temperature, T, must always be expressed on an absolute-temperature scale (K) cal/mol K 1.987 The quantity of gas, n, is normally expressed in moles J/mol K 8.314 m3 Pa/mol K 8.314 L atm/mol K The units chosen for pressure and volume are typically atmospheres (atm) and liters (l), however, other units may be chosen have the units of energy: PV can question : what is the pressure exerted by 0.508 mol O2 in a 15.0L container at 303 K? question : what is the mass of propane, C3H8 in a 50.0L container of the gas at STP? Molar Mass and Gas Densities PV = nRT and m m , n= d= M V m RT PV = M m =d= V MP RT question: calculate the molar mass of a liquid that vaporized at 100°C and 755 Torr yields 185mL of vapor with a mass 0.523g Mixtures of Gases Dalton’s law The total pressure of a mixture of gases equals the sum of the pressures that each would exert if it were present alone. Pt is the total pressure of a sample which contains a mixture of gases P1, P2, P3, etc. are the partial pressures of the gases in the mixture Partial pressure – Each component of a gas mixture exerts a pressure that it would exert if it were in the container alone. The partial pressure of a gas is equal to its mole fraction times the total pressure the term (X1 = n1 / nt ) is the mole fraction of a substance in the gaseous mixture. The mole fraction of a component expresses the ratio of the number of moles of one component to the total number of moles in the mixture. question : A gaseous mixture made from 10 g of oxygen and 15 g of methane is placed in a 10.0 L vessel at 25°C. What is the partial pressure of each gas, and what is the total pressure in the vessel? question : the main component of dry air by volume N2 78.08%, O2 20.95% Ar 0.93% and CO2 0.04%. what are the partial pressures of each of the four gases in a sample of air at 1.00atm. question : the total pressure of a gas mixture which containing 0.2 mol of CH4, 0.3 mol of N2 and 0.5 mol of H2, is 2atm. Calculate the partial pressures of each gases in atm. • Question: the reaction of aluminum with HCl produces Hydrogen gas, Al (s) + HCl (aq) → AlCl3 (aq) + H2 (g) if 35.5ml of H2 is collected over water at 26 °C and barometric pressure of 755.0mmHg, how many moles of HCl must have been consumed Pwater= at 26 C is 25.2mmHg 0,37gr KClO3 was heated and O2 produced in this reaction wwas collected over water. The temperatuer of water is 23°C ve atmosferic pressure is 751mmHg. What is the volume of O2 collected over water. Vapor pressure of water at 23°C is 21,1mmHg P tot= P gas + P H2O Kinetic Molecular Theory • Particles are point masses in constant, random, straight line motion. • Particles are separated by great distances. the actual volume of molecules is negligible. • Collisions are rapid and elastic. • No force between particles. • Total energy remains constant. • The average kinetic energy of the molecules is proportional to absolute temperature • Translational kinetic energy, 1 e k mu 2 2 • Frequency of collisions, N vu V • Impulse or momentum transfer, I mu • Pressure proportional to impulse times frequency N P mu 2 V • Three dimensional systems lead to: P 1N m u2 3V 1 2 PV N A m u 3 3RT N A m u 3RT M u u rms 2 3RT M 2 Molecular Speed The root mean square speed urms u rms 3RT M Units for R must be 8.314 joule mol/K for M must be in kg question: determine the urms of O2 and H2 at 0C. Diffusion is the process of the mixing of gases with one another. Each gas spreads throughout the mixture until its partial pressure is the same everywhere Effusion is a process in which a gas escapes from its container through a tiny hole. At a given temperature, the rates of effusion of a gas molecules are inversely proportional to the square roots of their molar masses. Effusion time and rates are inversely related. rate of effusion of A (u rms ) A 3RT/M A MB rate of effusion of B (u rms ) B 3RT/M B MA Ratio used can be: – Rate of effusion (as above) – Distances traveled by molecules – Molecular speeds – Amounts of gas effused. – Effusion times question: if an unknown gas has a effusion rate 0.468 times the rate of O2 at the same temperature, what is the molecular weight of the unknown gas? rate of effusion of A (u rms ) A 3RT/M A MB rate of effusion of B (u rms ) B 3RT/M B MA Nonideal (Real ) gases • An ideal gas is a gas in which the volumes of the molecules, intermolecular attractive forces and the loss of kinetic energy in collisions are neglected. • Compressibility factor PV/nRT=1 for ideal gases. • Gases tend to behave ideally at high temperatures and low pressures, and tend to behave nonideally at low temperatures and high pressures. Deviations occur for real gases. PV/nRT > 1 - molecular volume is significant. PV/nRT < 1 - intermolecular forces of attraction. Substance a (L2 atm/mol2) He 0.0341 0.0237 H2 0.244 0.0266 O2 1.36 0.0318 b(L/mol)