Alchemy Investigation IV
advertisement

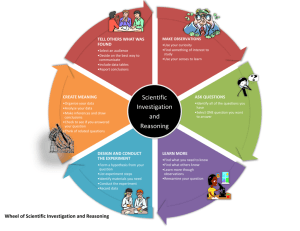
Alchemy Unit Investigation IV: Subatomic World Lesson 1: Island of Stability Lesson 2: It’s Greek to Me Lesson 3: ELEMENTary Education QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Alchemy Unit – Investigation IV Lesson 1: Island of Stability QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. ChemCatalyst The isotope notation for an atom of copper and an atom of gold are given below. 63 197 Cu 29 79 Au • How could you change a copper atom into a gold atom? • What would you need to change? Give specific numbers. • Why is this change called a nuclear reaction? © 2004 Key Curriculum Press. Unit 1 • Investigation IV The Big Question • What is the range of the number of neutrons found in isotopes of various elements? © 2004 Key Curriculum Press. Unit 1 • Investigation IV You will be able to: • Determine how many neutrons are required to make a stable element with a given number of protons. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Notes • Nuclear chemistry is the study of the nucleus of the atom. • The band of stability is the range in the number of neutrons for a given number of protons for isotopes that are found in nature. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Activity Purpose: Some combinations of neutrons, electrons, and protons are not stable enough to be called elements. This lesson shows you how to predict the numbers of neutrons, electrons, and protons of the isotopes they are likely to find in nature. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation IV (cont.) (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation IV Making Sense • What kind of generalization can you make about how the number of protons and neutrons are related to each other in the elements? © 2004 Key Curriculum Press. Unit 1 • Investigation IV Notes • Radioactive elements are less stable because the isotope is lost over time as pieces of the nucleus spontaneously emerge. • The concept of isotope stability is highly dependent on time. Atoms that exist for a long time are referred to as stable. Radioactive atoms disappear over time and are referred to as (cont.) unstable. © 2004 Key Curriculum Press. Unit 1 • Investigation IV (cont.) • Any isotope that is around long enough to be detected and measured qualifies as an element, but still can be highly unstable and radioactive. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Check-In • Use your graph to determine how many neutrons you would need to make a stable element with 75 protons. • How many neutrons would make a radioactive element with 75 protons? © 2004 Key Curriculum Press. Unit 1 • Investigation IV Wrap-Up • In order for an atom to be considered an element, it has to have a stable nucleus and exist long enough to be detected. • The neutron to proton ratio is an important factor in determining the stability of a nucleus. • Some isotopes are more stable than others. Unstable isotopes undergo nuclear decay to produce atoms with lower mass. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Alchemy Unit – Investigation IV Lesson 2: It’s Greek to Me QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. ChemCatalyst Uranium-238 is used in nuclear reactors to generate electricity. In the nuclear reactor, uranium-238 changes to lead-209. 238 92 U 209 82 Pb • How can atoms of uranium-238 change into atoms of lead-209? • The nuclear reaction is initiated by colliding the uranium-238 with 01 n . What do you think this symbol represents? © 2004 Key Curriculum Press. Unit 1 • Investigation IV The Big Question • What changes in the nucleus during radioactive decay? © 2004 Key Curriculum Press. Unit 1 • Investigation IV You will be able to: • Predict the result of radioactive decay of an atom. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Notes • A nuclear reaction happens when the nucleus of an atom is unstable and spontaneously decays emitting particles. • There are two types of nuclear decay, alpha and beta. Depending on the type of decay either an alpha particle or beta particle is emitted. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation IV (cont.) • Chemists use equations like the following one to represent nuclear reactions. 47 Ca 20 238 U 92 g g + 47 Sc 21 + 234 Th 90 © 2004 Key Curriculum Press. Unit 1 • Investigation IV Activity Purpose: This activity introduces you to two common forms of nuclear decay. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Making Sense • Give a specific example of how a chemist might make gold using alpha decay. Be specific about which isotope of gold is made. • Give a specific example of how a chemist might make gold using beta decay. Be specific about which isotope of gold is made. • Would the isotopes of gold prepared by alpha and beta decay be located in the band of stability? © 2004 Key Curriculum Press. Unit 1 • Investigation IV Notes • Alpha decay and beta decay are two forms of radiation or nuclear decay. • During alpha decay a nucleus is splitting into two smaller elements, one of which is always a helium atom. • Chemists use the symbol (the Greek letter alpha) to represent an alpha particle. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation IV (cont.) • During beta decay a neutron inside the nucleus of an atom emits an electron. This electron is a part of nuclear decay and therefore comes from the nucleus. • Under certain circumstances each neutron can be further split up into an electron and a proton. • Removal of an electron from a neutron alters the neutron so that it becomes a (cont.) proton. © 2004 Key Curriculum Press. Unit 1 • Investigation IV (cont.) • The process of splitting a large nucleus into smaller ones is called nuclear fission. • Besides alpha and beta particles, many radioactive nuclei release energy in the form of gamma rays ( rays). (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation IV (cont.) • The release of a gamma ray causes no change to either the mass number or the atomic number of an atom because a gamma ray has no mass. • Gamma radiation by itself does not change the identity of the atom. However, gamma ray emission usually occurs whenever there is alpha or beta emission. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Check-In • What products do you expect if an atom of actinium-227 undergoes alpha decay? © 2004 Key Curriculum Press. Unit 1 • Investigation IV Wrap-Up • When changes occur in the nucleus of an atom it is called a nuclear reaction. • When an alpha particle is emitted from an atom, the nucleus loses two protons and two neutrons. An alpha particle is the same as a helium atom. • When a beta particle is emitted from an atom, the nucleus gains a proton and loses a neutron. A beta particle is the same as an electron. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Alchemy Unit – Investigation IV Lesson 3: ELEMENTary Education QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. ChemCatalyst The periodic table lists every element after uranium, U, as “human-made” elements. • How are elements made in nature and by scientists? © 2004 Key Curriculum Press. Unit 1 • Investigation IV The Big Question • How are elements formed? © 2004 Key Curriculum Press. Unit 1 • Investigation IV You will be able to: • Explain what it would take to turn one element into another. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Activity Purpose: The goal of this lesson is to examine the formation of the elements. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Making Sense • Why are the small owls breaking out their digging tools at the end of the comic strip? © 2004 Key Curriculum Press. Unit 1 • Investigation IV Notes • The process of element formation is called nucleosynthesis. • Nuclear fusion is a process that produces bigger elements from smaller ones. It requires extraordinarily high temperatures. At such high temperatures, nuclei are moving so fast that collisions between them can overcome the natural repulsion of their positive charges. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation IV (cont.) • Heavier elements do not burst into fiery fusion by combination with -particles. They are formed by neutron capture followed by beta decay, among other processes. Nuclei add neutrons until one is converted into a proton and an electron (beta decay). A new element is formed because the atomic number increases by one. This happens in supernova explosions. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation IV (cont.) • Chemical reactions are ones in which atoms remain unchanged. • Revised definition of an element: Stable elements cannot be broken apart into new elements in chemical reactions. It is possible to convert one element into another in nuclear reactions, but this requires a lot of energy for stable elements. Unstable elements undergo radioactive decay, and are broken apart spontaneously. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Check-In Consider the chemical reaction between hydrogen and oxygen to form water. H2 + 2O2 2H2O • Name two ways in which a chemical reaction is different from a nuclear reaction. © 2004 Key Curriculum Press. Unit 1 • Investigation IV Wrap-Up • Elements are converted from one to another in nuclear reactions, but not in chemical reactions. • Nuclear reactions involve the energy of a supernova. © 2004 Key Curriculum Press. Unit 1 • Investigation IV