316354
advertisement

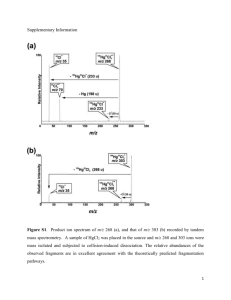
Matrix Isolation and Computational Study of [2C, 2N, X] (X = S, Se) Isomers Tamás Vörös, György Tarczay Institute of Chemistry, Eötvös University Budapest, Hungary Outline of the presentation • Introduction, goals of our studies • Computational results • Experimental results • Summary Outline of the presentation • Introduction, goals of our studies • Computational results • Experimental results • Summary Introduction • Early 19th century: Wöhler: Ag-cyanate (AgOCN) Isomerism (Berzelius) Liebig: Ag-fulminate (AgCNO) • Presently: HNCX HXCN HCNX HXNC X=O X=S X = Se ! ! ! Introduction Sgr B2: HNCO HOCN HNCS HSCN TCM-1: HCNO Introduction NCNCX NCCNX NCXCN NCXNC X=O ! ! X=S X = Se [1] AgCN + SCl2 NCSCN + 2 AgCl [2] AgNCSe + I2 NCSeSeCN + 2 AgI NCSeCN + (NCSe)2Se [1a] C. J. Burchell, P. Kilian, A. M. Z. Slawin, J. D. Woolins; Inorg. Chem., 45 (2006) 710-716. [1b] Z. Kisiel et al.; J. Phys. Chem. A, 117 (2013) 13815-13824. [2] F. Cataldo, Polyhedron, 19 (2000) 681-688. Goals of our studies To study the [2C, 2N, X] (X = S, Se) isomers • using quantum-chemical methods to compute: - equilibrium structures, relative energies - harmonic and anharmonic wavenumbers, IR intensities and UV excitation energies • using matrix-isolation technique to: - supplement the condensed-phase IR spectra of NCXCN - generate and spectroscopically identify new isomers from the NCXCN isomer Outline of the presentation • Introduction, goals of our studies • Computational results • Experimental results • Summary Equilibrium structuresa, relative energiesb a CCSD(T)/aug-cc-pVTZ b ΔE (CCSD(T)/aug-cc-pVTZ) + ΔZPVE (CCSD(T)/aug-cc-pVTZ) Equilibrium structures NCCNO CNCNS NCNCSe MP2/6-31G* [3] bent, quasilinear bent linear CCSD(T)/ aug-cc-pVTZ linear linear bent [3] M. Feher, T. Pasinszki, T. Veszpremi, Inorg. Chem., 34 (1995) 945-951. IR wavenumbers and intensitiesa – [2C, 2N, S] NCNCS NCSCN NCCNS NCSNC NCC(NS) 2247 (459) 2181 (0.3) 2229 (719) 2161 (0.7) 2238 (12) 2013 (1229) 2171 (0.1) 2085 (274) 2033 (217) 1705 (1) 1177 (17) 650 (2) 1073 (100) 679 (11) 961 (50) 658 (3) 649 (7) 558 (32) 630b (20) 631 (4) 470 (40) 487 (1) 377 (0.005) 457b (3) 513 (4) 447 (3) 359 (0) 372 (15) 358 (2) 506 (0.1) 442 (10) 349 (4) 77 (10) 242 (0.01) 363 (10) 423 (7) 309 (2) 239 (2) 220 (15) 84 (4) 120 (8) 110 (6) 164 (6) a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharm. contributions b) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/cc-pVDZ anharm. contributions (IR intensities: harmonic CCSD(T)/aug-ccpVTZ) IR wavenumbers and intensities – [2C, 2N, S] CNNCSa CNCNSb CNSNCa CNC(NS)a 2097 (4) 2291 (418) 2032 (86) 2084 (419) 1891 (1040) 2010 (302) 2003 (371) 1698 (10) 1094 (21) 1108 (147) 693 (23) 1038 (107) 719 (18) 564 (36) 685 (47) 668 (7) 522 (42) 349 (6) 414 (7) 486 (14) 418 (0.2) 266 (0.002) 255 (0.00) 479 (6) 308 (9) 70 (11) 244 (0.06) 327 (2) 273 (1) 195 (0.08) 170 (5) 111 (3) 105 (4) 143 (3) a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharm. contributions b) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers (IR intensities: harmonic CCSD(T)/aug-ccpVTZ) IR wavenumbers and intensitiesa – [2C, 2N, Se] NCNCSe NCSeCN NCCNSe NCSeNC NCC(NSe) 2257 (617) 2176 (2) 2222 (817) 2160 (2) 2233 (10) 2012 (1232) 2168 (1) 2088 (340) 2039 (241) 1702 (4) 1106 (16) 547 (6) 1003 (65) 551 (17) 930 (48) 510 (3) 528 (6) 406 (29) 533 (14) 587 (14) 434 (28) 439 (1) 393 (0.02) 406 (2) 501 (0.01) 429 (8) 330 (0.0) 352 (21) 325 (2) 434 (1) 404 (17) 316 (3) 94 (9) 220 (0.004) 350 (12) 387 (0.3) 282 (1) 215 (2) 217 (14) 65 (5) 104 (7) 97 (5) 142 (5) a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharm. contributions b) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/cc-pVDZ anharm. contributions (IR intensities: harmonic CCSD(T)/aug-ccpVTZ) IR wavenumbers and intensitiesa – [2C, 2N, Se] CNNCSe CNCNSe CNSeNC CNC(NSe) 2109 (7) 2226 (461) 2040 (120) 2092 (426) 1859 (1032) 1972 (305) 2017 (396) 1702 (6) 1023 (30) 1033 (109) 565 (17) 1002 (113) 619 (42) 369 (30) 549 (43) 605 (24) 444 (36) 334 (15) 346 (6) 457 (4) 380 (0.02) 275 (0.5) 229 (0.0) 420 (8) 299 (9) 96 (8) 219 (0.05) 303 (2) 270 (1) 179 (0.05) 172 (5) 99 (4) 94 (4) 132 (2) a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharm. contributions (IR intensities: harmonic CCSD(T)/aug-ccpVTZ) Outline of the presentation • Introduction, goals of our studies • Computational results • Experimental results • Summary Preparation of NCSCN 2 AgCN + SCl2 = NCSCN + 2 AgCl [1a] [1b] Solvent 40 cm3 CH2Cl2 200 cm3 CS2 Amounts of the reactants 1.813 g AgCN, 0.4 cm3 SCl2 26.5 g AgCN, 10.0 cm3 SCl2 Time of the reaction 60 min 30 min Temperature during the reaction 0 °C 30 °C [1a] C. J. Burchell, et al., Inorg. Chem., 45 (2006) 710-716. [1b] Z. Kisiel et al.; J. Phys. Chem. A, 117 (2013) 13815-13824. MI-IR spectra of NCSCN a) in argon b) in krypton c) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-ccpVTZ) MI-IR spectra of NCSCN Experimental Computeda KBr pellet [2] Assignment Ar matrix Kr matrix KBr pellet [1a] 2666 (0.3) 2681.4 (2) - - - ν3 + ν1 2527 (0.3) 2542.2 (3) 2538.3 (2) - - ν6 + ν1 2527 (0.4) 2540.9 (2) 2536.2 (3) - - ν7 + ν5 2487 (0.2) 2502.1 (1) 2498.3 (1) - - ν9 + ν1 2476 (0.2) 2491.3 (2) 2487.3 (1) - - ν9 + ν7 2185 (0.3) 2192.9 (2), 2190.2 (2) 2188.9 (3) - - ν1 CN str. 2171 (0.1) 2181.9 (4), 2178.8 (7) 2177.8 (8) 2184 vs 2180 s ν7 CN str. 967 (0.1) 948.6 (3) - - - ν9 + ν2 651 (2) 680.1 (100) 678.3 (100) 697 m 685 m ν8 CS str. 650 (7) 677.1 (56) 676.3 (52) 670 m - ν2 CS str. 487 (1) - - - 465 w ν3 SCN bend. a (CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-ccpVTZ)) Photolysis of NCSCN in argon a) 254 nm photo. – deposited b) BBUV – 254 nm photo. c) and d) NCNCS and NCSNC (CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/augccpVTZ)) Photolysis of NCSCN in argon A A0 1 e kt Wavenumber / cm–1 k / min–1 1185.0 0.00063 ± 0.00002 1994.9 0.00065 ± 0.00002 2256.6 0.00065 ± 0.000005 2367.4 0.00060 ± 0.00006 2689.6 0.00065 ± 0.00004 2045.7 0.00106 ± 0.000009 690.8 0.00097 ± 0.00006 673.1 0.00101 ± 0.00003 Photolysis of NCSCN in krypton a) 254 nm photo. – deposited b) BBUV – 254 nm photo. c) and d) NCNCS and NCSNC (CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/augccpVTZ)) Photolysis of NCSCN: NCSNC Computeda Experimental Assignment Ar matrix Kr matrix 2161 (0.7) - - ν1 CN str. 2033 (217) 2045.7 (68), 2043.4 (32)b 2043.1 (100) ν2 NC str. 750 (14) - - 2ν8 728 (28) 690.8 (12) 691.2 (1) ν9 + ν5 679 (11) 673.1 (4) 689.1 (2) ν4 NS str. 630 (20) - - ν3 SC str. 457 (3) - - ν5 SCN bend. 358 (2) c c ν8 SCN bend. 242 (0.01) c c ν9 SNC bend. 239 (2) c c ν6 SNC bend. 110 (6) c c ν7 NSN def. a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-cc-pVTZ) b) Site split bands. c) Not in the measured spectral region. Photolysis of NCSCN: NCNCS Computeda Experimental Assignment Ar matrix Kr matrix gas [d] gas [e] 2675 (38) 2689.6 (3) 2683.1 (3) - - ν4 + ν2 2355 (89) 2367.4 (2) 2368.0 (3) - - 2ν3 2247 (459) 2256.6 (32) 2251.7 (42) 2260.9 2240 ν1 NC str. 1995.8 (100) 2016.4 1920 ν2 NC str. 2013 (1229) 1994.9 (94), 1992.1 (6)b 1177 (17) 1185.0 (0.7) 1185.6 (0.8) - 1105 ν3 CS str. 658 (3) - - - - ν4 CN str. 470 (40) - - - - ν5 NCN bend. 447 (3) - - - - ν8 NCN bend. 442 (10) - - - - ν6 NCS bend. 423 (7) - - - - ν9 NCS bend. 84 (4) c c c c ν7 CNC def. a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-cc-pVTZ) b) Site split bands. c) Not in the measured spectral region. d) DeVore, T. C. J. Mol. Struct. 1987, 162, 287. e) Neidlein R.; Reuter, H. G. Arch. Pharm. 1975, 308, 189. Preparation of NCSeCN [2] a) KNCSe + CH3COOAg = AgNCSe + CH3COOK b) AgNCSe + I2 = NCSeSeCN + 2 AgI 2 NCSeSeCN = NCSeCN + (NCSe)2Se Raman spectrum of NCSeCN: [2] F. Cataldo, Polyhedron, 19 (2000) 681-688. MS spectrum of NCSeCN: MI-IR spectra of NCSeCN a) in argon b) in krypton c) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-ccpVTZ) MI-IR spectra of NCSeCN Comp.a Experimental Assignment Ar Kr KBr pellet [d] 2704 (0.1) 2699.3 (1) - - ν2 + ν1 2613 (0.2) 2619.6 (1) 2617.7 (2) - ν3 + ν1 2495 (0.4) 2510.2 (0.7) 2499.8 (1) - ν7 + ν5 2489 (0.3) 2502.6 (4) 2497.0 (0.8) - ν6 + ν1 2455 (0.1) 2464.7 (1) 2462.0 (1) - ν9 + ν1 2447 (0.2) 2455.7 (2) 2453.4 (1) - ν9 + ν7 2176 (2) 2186.3 (5), 2183.1 (20)b 2182.7 (5), 2181.6 (10)b 2183 m ν1 CN str. 2168 (1) 2174.0 (10) 2173.9 (2), 2172.3 (4)b 2175 m ν7 CN str. 581 (5) 573.6 (14) 573.1 (11) - 2ν9 547 (6) 527.5 (1), 526.1 (62), 524.4 (37)b 523.0 (18), 524.2 (21), 525.4 (35), 526.7 (26)b 516 vs ν8 CSe str. 528 (6) 522.6 (17), 521.0 (1)b 520.8 (14), 519.3 (1)b - ν2 CSe str. 439 (1) - - 436 m ν3 SeCN b. a) b) c) d) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-cc-pVTZ) Site split bands. Not in the measured spectral region. E. E. Aynsley, N. N. Greenwood, J. Sprague; J. Chem. Soc., (1964) 704. Photolysis of NCSeCN in argon a) 254 nm photo. – deposited b) BBUV – 254 nm photo. c) and d) NCNCS and NCSNC (CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/augccpVTZ)) Photolysis of NCSeCN in krypton a) 254 nm photo. – deposited b) BBUV – 254 nm photo. c) and d) NCNCS and NCSNC (CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/augccpVTZ)) Photolysis of NCSeCN: NCSeNC Computeda Experimental Assignment Ar matrix Kr matrix 2160 (2) - - ν1 CN str. 2039 (241) 2055.3 (49), 2049.6 (51)b 2047.5 ν2 NC str. 551 (17) - - ν3 SeC str. 533 (14) - - ν4 NSe str. 406 (2) - - ν5 SeCN bend. 325 (2) c c ν8 SeCN bend. 220 (0.004) c c ν9 SeNC bend. 215 (2) c c ν6 SeNC bend. 97 (5) c c ν7 NSeN def. a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-cc-pVTZ) b) Site split bands. c) Not in the measured spectral region. Photolysis of NCSeCN: NCNCSe Computeda Experimental Kr matrix Assignment 2257 (617) 2260.9 (52) ν1 NC str. 2012 (1232) 1970.7 (89), 1965.7 (11) ν2 NC str. 1106 (16) - ν3 CSe str. 510 (3) - ν4 CN str. 434 (28) - ν8 NCN bend. 429 (8) - ν5 NCN bend. 404 (17) b ν6 NCSe bend. 387 (0.3) b ν9 NCSe bend. 65 (5) b ν7 CNC def. a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-cc-pVTZ) b) Not in the measured spectral region. Outline of the presentation • Introduction, goals of our studies • Computational results • Experimental results • Summary Summary NCNCX NCCNX NCXCN NCXNC X=O X=S X = Se ! ! ! ! ! Thank you for your attention! Instruments Matrix-isolation setup: • Lowest temperature: 8 K • Cryostat: Closed-cycle He (Air Products Displex DE 202) • Windows: CsI (for IR), NaCl (for UV) IR spectrometer: • • • • Type: IFS 28 FT-IR 125 W high-pressure Source: Globar Hg lamp + Detector: DTGS Best resolution: 1 Lamp: Cathodeon HPK cm-1 Melles Griot interference filters UV excitation energiesa – [2C, 2N, S] NCNCS NCSCN NCCNS NCSNC NCC(NS) CNNCS CNCNS CNC(NS) CNSNC 286 (0.0000) 231 (0.0016) 364 (0.0000) 267 (0.0003) 456 (0.0040) 343 (0.0003) 354 (0.0000) 485 (0.0005) 281 (0.0000) 261 (0.0001) 206 (0.0002) 354 (0.0000) 203 (0.0019) 345 (0.0024) 262 (0.0028) 347 (0.0000) 313 (0.0040) 215 (0.0032) 213 (1.3034) 249 (0.0007) 218 (0.0005) 226 (1.1834) 237 (0.0003) 206 (0.0048) 235 (0.0000) 224 (0.0054) 218 (0.0003) 209 (0.0483) 201 (0.0416) 213 (0.0501) 245 (0.0001) a) EOMEE-CCSD/aug-cc-pVTZ excitation energies (oscillator strengths: TD-DFT B3LYP/aug-cc-pVTZ) UV excitation energiesa – [2C, 2N, Se] NCNCSe NCSeCN NCCNSe NCSeNC NCC(NSe) CNNCSe CNCNSe CNC(NSe) CNSeNC 311 (0.0000) 255 (0.0000) 395 (0.0000) 200 (0.0002) 263 (0.0003) 220 (0.0001) 206 (0.0000) 273 (0.0003) 313 (0.0000) 290 (0.0001) 212 (0.0026) 388 (0.0000) 203 (0.0000) 247 (0.0000) 201 (0.0000) 276 (0.0001) 245 (1.0828) 206 (0.0762) a) EOMEE-CCSD/aug-cc-pVTZ excitation energies (oscillator strengths: TD-DFT B3LYP/aug-cc-pVTZ) 232 (0.0001) Raman wavenumbers and intensitiesa – [2C, 2N, S] NCNCS NCSCN NCCNS NCSNC NCC(NS) 2247 (411) 2181 (100) 2229 (516) 2161 (81) 2238 (139) 2013 (6) 2171 (30) 2085 (19) 2033 (68) 1705 (59) 1177 (26) 650 (22) 1073 (1) 679 (23) 961 (47) 658 (75) 649 (1) 558 (79) 630b (15) 631 (66) 470 (16) 487 (19) 377 (2) 457b (23) 513 (5) 447 (12) 359 (8) 372 (59) 358 (6) 506 (41) 442 (23) 349 (4) 77 (61) 242 (18) 363 (126) 423 (2) 309 (0.1) 239 (7) 220 (9) 84 (273) 120 (190) 110 (228) 164 (95) a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharm. contributions b) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/cc-pVDZ anharm. contributions (Raman intensities: B3LYP/aug-cc-pVTZ) Raman wavenumbers and intensities – [2C, 2N, S] CNNCSa CNCNSb CNSNCa CNC(NS)a 2097 (352) 2291 (532) 2032 (147) 2084 (171) 1891 (5) 2010 (432) 2003 (45) 1698 (51) 1094 (32) 1108 (1) 693 (27) 1038 (45) 719 (23) 564 (62) 685 (4) 668 (61) 522 (94) 349 (28) 414 (31) 486 (50) 418 (0.03) 266 (91) 255 (25) 479 (2) 308 (38) 70 (15) 244 (5) 327 (105) 273 (33) 195 (8) 170 (18) 111 (336) 105 (259) 143 (101) a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharm. contributions b) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers (Raman intensities: B3LYP/aug-cc-pVTZ) Raman wavenumbers and intensitiesa – [2C, 2N, Se] NCNCSe NCSeCN NCCNSe NCSeNC NCC(NSe) 2257 (531) 2176 (95) 2222 (650) 2160 (80) 2233 (165) 2012 (10) 2168 (34) 2088 (18) 2039 (55) 1702 (54) 1106 (13) 547 (46) 1003 (1) 551 (83) 930 (56) 510 (122) 528 (7) 406 (91) 533 (35) 587 (57) 434 (15) 439 (22) 393 (64) 406 (18) 501 (4) 429 (0.7) 330 (8) 352 (0.3) 325 (7) 434 (87) 404 (0.9) 316 (8) 94 (4) 220 (31) 350 (137) 387 (6) 282 (0.3) 215 (7) 217 (15) 65 (3) 104 (206) 97 (276) 142 (110) a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharm. contributions b) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/cc-pVDZ anharm. Contributions (Raman intensities: B3LYP/aug-cc-pVTZ) Raman wavenumbers and intensitiesa – [2C, 2N, Se] CNNCSe CNCNSe CNSeNC CNC(NSe) 2109 (467) 2226 (702) 2040 (112) 2092 (211) 1859 (9) 1972 (565) 2017 (42) 1702 (48) 1023 (24) 1033 (5) 565 (105) 1002 (43) 619 (11) 369 (65) 549 (27) 605 (88) 444 (142) 334 (76) 346 (20) 457 (1) 380 (0.07) 275 (33) 229 (38) 420 (84) 299 (44) 96 (16) 219 (14) 303 (106) 270 (41) 179 (14) 172 (26) 99 (398) 94 (325) 132 (124) a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharm. contributions (Raman intensities: B3LYP/aug-cc-pVTZ) Relative Raman intensities 4 ~ ~ ~ ~ I i f ( 0 i ) S i / i 1 exp hc i / kT V. Krishnakumar, G. Keresztury, T. Sundius, R. Ramasamy, J. Mol. Struct. 2004, 702, 9–21. Experimental (NCSCN) Computeda KBr pellet [6] Assignment Ar matrix Kr matrix KBr pellet [5a] 2666 (0.3) 2681.4 (2) - - - ν3 + ν1 2527 (0.3) 2542.2 (3) 2538.3 (2) - - ν6 + ν1 2527 (0.4) 2540.9 (2) 2536.2 (3) - - ν7 + ν5 2487 (0.2) 2502.1 (1) 2498.3 (1) - - ν9 + ν1 2476 (0.2) 2491.3 (2) 2487.3 (1) - - ν9 + ν7 2185 (0.3) 2192.9 (2), 2190.2 (2) 2188.9 (3) - - ν1 CN str. 2171 (0.1) 2181.9 (4), 2178.8 (7) 2177.8 (8) 2184 vs 2180 s ν7 CN str. 967 (0.1) 948.6 (3) - - - ν9 + ν2 651 (2) 680.1 (100) 678.3 (100) 697 m 685 m ν8 CS str. 650 (7) 677.1 (56) 676.3 (52) 670 m - ν2 CS str. 487 (1) - - - 465 w ν3 SCN bend. 359 (0.0) - - - - ν5 SCN bend. 349 (4) - - 379 m - ν6 SCN bend. 309 (2) - - - - ν9 SCN bend. 120 (8) ν4 CSC def. a (CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-ccpVTZ)) Photolysis of NCSCN in krypton A A0 1 e kt Wavenumber / cm–1 k / min–1 1185.6 0.00066 ± 0.00002 1995.8 0.00066 ± 0.00002 2251.7 0.00052 ± 0.00002 2368.0 0.00068 ± 0.00004 2683.1 0.00069 ± 0.00006 2043.1* 0.00145 ± 0.00005 * Fitted only in the 0 – 2040 min region. Emission spectrum of S2 MI-UV spectrum of NCSCN NCSeCN Observed Comp.a Assignment Ar Kr KBr pellet [6] KBr pellet [d] 2704 (0.1) 2699.3 (1) - - - ν2 + ν1 2613 (0.2) 2619.6 (1) 2617.7 (2) - - ν3 + ν1 2495 (0.4) 2510.2 (0.7) 2499.8 (1) - - ν7 + ν5 2489 (0.3) 2502.6 (4) 2497.0 (0.8) - - ν6 + ν1 2455 (0.1) 2464.7 (1) 2462.0 (1) - - ν9 + ν1 2447 (0.2) 2455.7 (2) 2453.4 (1) - - ν9 + ν7 2176 (2) 2186.3 (5), 2183.1 (20)b 2182.7 (5), 2181.6 (10)b 2181 s 2183 m ν1 CN str. 2168 (1) 2174.0 (10) 2173.9 (2), 2172.3 (4)b - 2175 m ν7 CN str. 581 (5) 573.6 (14) 573.1 (11) - - 2ν9 547 (6) 527.5 (1), 526.1 (62), 524.4 (37)b 523.0 (18), 524.2 (21), 525.4 (35), 526.7 (26)b 507 vvs 516 vs ν8 CSe str. 528 (6) 522.6 (17), 521.0 (1)b 520.8 (14), 519.3 (1)b - - ν2 CSe str. 439 (1) - - 436 w 436 m ν3 SeCN b. 330 (0) - - - 345 w ν5 SeCN b. 316 (3) - - - 336 s ν6 SeCN b. 282 (1) - - - 312 w ν9 SeCN b. c c 104 (7) a) CCSD(T)/aug-cc-pVTZ harmonic wavenumbers + CCSD(T)/aug-cc-pVDZ anharmonic contributions (IR intensities: harmonic CCSD(T)/aug-cc-pVTZ) b) Site split bands. c) Not in the measured spectral region. d) E. E. Aynsley, N. N. Greenwood, J. Sprague; J. Chem. Soc., (1964) 704. ν4 CSeC def. References – [H, C, N, X] [3a] M.E. Jacox, D.E. Milligan, J. Chem. Phys. 40 (1964) 2457. [3b] V.E. Bondybey, J.H. English, C.W. Mathews, R.J. Contolini, J. Mol. Spectrosc. 92 (1982) 431. [3c] J.H. Teles, G. Maier, B.A. Hess, L.J. Schaad, M. Winnewisser, B.P. Winnewisser, Chem. Ber. 122 (1989) 753. [3d] J.N. Crowley, J.R. Sodeau, J. Phys. Chem. 93 (1989) 3100. [3e] M. Pettersson, L. Khriachtchev, S. Jolkkonen, M. Räsänen, J. Phys. Chem. A 103 (1999) 9154. [3f] M. Mladenovic, M. Lewerenz, M.C. McCarthy, P. Thaddeus, J. Chem. Phys. 131 (2009) 174308. [3g] M. Wierzejewska, J. Moc, J. Phys. Chem. A 107 (2003) 11209. [3h] J.R. Durig, D.W. Wertz, J. Chem. Phys. 46 (1967) 3069. [3i] M. Wierzejewska, Z. Mielke, Chem. Phys. Lett. 349 (2001) 227. [3j] T. Pasinszki, M. Krebsz, G. Bazsó, G. Tarczay, Chem. Eur. J. 15 (2009) 6100. [3k] B. M. Landsberg, Chem. Phys. Lett. 60 (1979) 265. [3l] J. Vogt, M. Winnewisser, Ber. Bunsen-Ges. 88 (1984) 439. [3m] J. Vogt, M. Winnewisser, Ber. Bunsen-Ges. 88 (1984) 444. [3m] M. Krebsz, G. Májusi, B. Pacsai, G. Tarczay, T. Pasinszki, Chem.–Eur. J. 18 (2012) 2646. [3n] T. Vörös, G. Bazsó, G. Tarczay, J. Phys. Chem. A 117 (2013) 13616. References – [2C, 2N, 2X] [4a] T. Pasinszki, Phys. Chem. Chem. Phys. 10 (2008) 1411. [4b] A. Schulz, T.M. Klapötke, Inorg. Chem. 35 (1996) 4791. [4c] G. Maier, M. Naumann, H.P. Reisenauer, J. Eckwert, Angew. Chem. Int. Ed. 35 (1996) 1696. [4d] Ch. Grundmann, Angew. Chem. Int. Ed. 2 (1963) 260. [4e] Ch. Grundmann, V. Mini, J.M. Dean, H.-D. Frommeld, Justus Liebigs Ann. Chem. 687 (1965) 191. [4f] G. Maier, J.H. Teles, Angew. Chem. Int. Ed. 26 (1987) 155. [4g] T. Pasinszki, N.P.C. Westwood, J. Am. Chem. Soc. 117 (1995) 8425. [4h] B. Guo, T. Pasinszki, N.P.C. Westwood, P.F. Bernath, J. Chem. Phys. 103 (1995) 3335. [4i] T. Vörös, G. Bazsó, Gy. Tarczay, T. Pasinszki, J. Mol. Structure, 1025 (2012) 117. [4j] E. Söderbäck, Liebigs. Ann. Chem. 419 (1919) 21. [4k] F. Seel, D. Wesemann, Chem. Ber. 86 (1953) 1107. [4l] F. Seel, D. Wesemann, Chem. Ber. 88 (1955) 1747. [4m] F. Cataldo, J. Inorg. Organomet. Polym. 7 (1997) 35. [4n] A.J. Barnes, S. Suzuki, in: W.F.Murphy (Ed.), Proceedings of the 7th International Conference Raman Spectroscopy, Ottawa, 1980, North-Holland, Amsterdam, 1980, p. 186. [4o] F. Cataldo, Polyhedron 19 (2000) 681. [4p] C. J. Burchell et. al., Inorg. Chem. 45 (2006) 710. References – [2C, 2N, X] • • • • • • • • • • • • • • • • F. Cataldo, Polyhedron 19 (2000) 681. C. J. Burchell, et. al., Inorg. Chem. 45 (2006) 710. Feher, Miklos; Pasinszki, Tibor; Veszpremi, Tamas Inorg. Chem., 34 (1995) 945. W. H. Hocking and M. C. L. Gerry, J. Mol. Spectrosc., 59 (1976) 338. W. H. Hocking and M. C. L. Gerry, J. Chem. Sot. Chem. Commun., (1973) 47. B. Bak, H. Svanholt and A. Holm, Acta Chem. Stand., Ser. A., 33 (1979) 597. D. C. Frost, H. W. Kroto, C. A. McDowell and N. P. C. Westwood, J. Electron. Spectrosc. Relat. Phenom., 11 (1977) 147. Mayer, E, Monatsh. Chem., 101 (1970) 834. DeVore, T.C., J. Mol. Struct., 162 (1987) 287. Pasinszki, T.; Westwood, N.P.C., J. Phys. Chem., 42 (1996) 16586. Guo, B.; Pasinszki, T.; Westwood, N.P.C.; Zhang, K.; Bernath, P.F.,J. Chem. Phys., 105 (1996) 4457. Brupbacher, Th.; Bohn, R.K.; Jager, W.; Gerry, M.C.L.; Pasinszki, T., Westwood, N.P.C., J. Mol. Spectrosc., 181 (1997) 316. Maier, G.; Teles, J. H. Angew. Chem. 99 (1987) 152. Hand, C. W.; Hexter, R. M. J. Am. Chem. Soc. 92 (1970) 1828. Zbigniew Kisiel, Manfred Winnewisser, B. P. Winnewisser, F. C. De Lucia, D. W. Tokaryk, B. E. Billinghurst, J. Phys. Chem. A 117 (2013) 13815 References – [2C, 2N, X] • • • • • • • • • • • Cataldo, Franco; Keheyan, Yeghis; Polyhedron; 21 (2002) 1825. King, Michael A.; Kroto, Harold W.; J. Am. Chem. Soc. 106 (1984) 7347. Jemson, H. M.; Gerry, M. C. L.; J. Mol. Spectr. 124 (1987) 481. Il'in, A. P.; Eremin, L. P.; Il'ina, T. P.; Russian Journal of Inorganic Chemistry (Translation of Zhurnal Neorganicheskoi Khimii);26 (1981) 913. Klapotke, T. M.; Krumm, B.; Galvesz-Ruiz, J. C.; Noth, H.; Schwab, I. Eur. J. Inorg. Chem. 24 (2004) 4764. King, Michael A.; Kroto, Harold W., J. Chem. Soc., Chem. Comm. 13 (1980) 606. King, M.A.; Kroto, H.W.; Landsberg, B.M., J. Mol. Spectrosc., 113 (1985) 1. M. Krebsz, Gy. Tarczay, T. Pasinszki, Chem. Eur. J. 19 (2013) 17201. Aynsley et al.; J. Chem. Soc. (1964) 704. Linke, K. H.; Lemmer, F. Z. Anorg. Allg. Chem. 345 (1966) 211. M. Krebsz, G. Majusi, B. Pacsai, Gy. Tarczay, T. Pasinszki Chemistry, A European Journal 18 (2012) 2646.