chemical symbol
advertisement

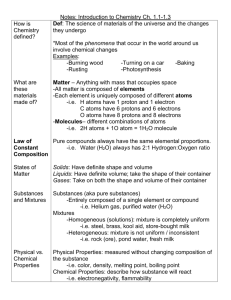
8th Grade Unit 3: Interactions of Matter and Energy Lesson 1: Chemical Reactions Vocabulary of Instruction: 1. Chemical Change • The atoms of a substance are rearranged into new substances with different chemical composition. • Evidence of a chemical change include: – color change. – odor change. – Bubbling. – fizzing (production of gas). – production of light or heat. – formation of a solid (precipitate). 2. Chemical Equation • A chemical equation consists of the chemical formulas of the substances that are mixed (called the reactants), and the chemical formula of the substances that result from the chemical reaction (called the products). The two are separated by an → — usually read as "yields“ — and each chemical formula is separated from others by a plus sign. Sometimes a triangle is drawn over the arrow symbol to denote energy must be added to the substances for the reaction to begin. • As an example, a reaction where hydrogen combines with oxygen to produce water can be denoted as follows: H2 + O → H2O • This can be read as "H two plus O, yields H two O". 2-A. Chemical Equation • A short, simple way to describe a chemical reaction using symbols and formulas instead of words. The substances to the left of the are called reactants and the substances to the right are called products. Example Equation for Photosynthesis: 6CO2 + 6H2O + Sunlight C6H12O6 + 6O2 + Heat Carbon dioxide + water + energy from the sun yields Glucose + Oxygen + Heat. 3. Chemical Formula • Combination of symbols and numbers that represent the elements in a compound. It shows the ratio of elements in a compound. Structural Formula C16H2N2Cl2 Chemical Formula 4. Chemical Reaction • Describes the interaction or combination between two or more substances that produces new substances, which characteristics are different from the original substances. 5. Chemical Symbol • A chemical symbol is an abbreviation or short representation of the name of a chemical element. Natural elements all have symbols of one or two letters. The first letter will always be Capitalized. For example: • • • • • • • H = Hydrogen Cl = Chlorine C = Carbon Mg = Magnesium Ag = Silver Au = Gold P = Phosphorus 5-A. Chemical Symbols Chart 6. Coefficient • A coefficient is the number of molecules of reactants or products in a chemical equation. – For example: • 2 H2 + O2 > 2 H2O means that two molecules of hydrogen react with one molecule of oxygen to produce two molecules of H2O (water). 6-A. Coefficient • A chemical formula may be preceded by a coefficient indicating the proportion of that substance necessary to produce the chemical reaction in a formula. For instance, the formula for the burning of methane » CH4 + 2O2 → CO2 + 2H2O indicates that twice as much O2 as CH4 is needed, and when they react will produce twice as much H2O as CO2. This is because during the reaction, each atom of carbon needs exactly two atoms of oxygen to combine with and to produce the CO2, and every two atoms of hydrogen need an atom of oxygen to combine with and to produce the H2O. • • 6-B. Coefficient Using Coefficients with Formulas You have learned that the subscript numbers in a chemical formula represent the number of atoms in one molecule or in one formula unit of an ionic compound. Now you will learn about the other numbers, called coefficients, that you often see to the left of a chemical formula. While a subscript number acts as a multiplier for a single element (unless there are parenthesis), a coefficient number acts as a multiplier for all of the atoms in the entire compound. As with subscripts, when no number is present then "1" is understood. • Look at the example below: • • CO2 Here we have one molecule of carbon dioxide. The subscript 2 in the formula above only pertains to the oxygen in the compound. The total number of atoms in the compound is 3. • – Now let us put a coefficient in front of the molecule and see how that changes things. • 5 CO2 The coefficient 5 refers to the entire molecule. It shows that there are 5 molecules of carbon dioxide. Since each molecule is made up of 3 atoms, the total number of atoms is now 15. – There are 5 carbon atoms and 10 oxygen atoms. 6-C. Coefficient • • • • • Using Coefficients with Formulas – Now, for an example with parenthesis: • Ba(NO3)2 Here we have one formula unit of the ionic compound, barium nitrate. We say "formula unit" instead of "molecule" because ionic compounds don't form molecules. The subscript 3 pertains to the oxygen, showing 3 oxygen atoms for each polyatomic ion of nitrate. The subscript 2 is a multiplier for everything in the parenthesis, because it is showing that there are two nitrate ions for every barium ion. The total number of atoms for each formula unit of barium nitrate is 9. There are: 1 barium atom, 2 nitrogen atoms, and 6 oxygen atoms. – Now let's put a coefficient in front of the formula unit and see how it changes the tally: • 3 Ba(NO3)2 Now we have 3 formula units of barium nitrate. The 3 coefficient acts as a multiplier for the entire compound. If there are 9 atoms in one formula unit of barium nitrate, then there must be 27 atoms in three formula units. There are: 3 barium atoms, 6 nitrogen atoms and 18 oxygen atoms. 7. Compound • A substance made of two or more elements that are chemically bound together in a set ratio. Examples: – Carbon dioxide • (CO2) – Water • (H2O) 8. Endothermic Reaction • Endothermic Chemical Reaction describes a chemical reaction that takes in heat energy. • Example: Photosynthesis - process in which plants use or take in the energy from the sun to convert carbon dioxide and water into glucose and oxygen. • The Chemical Reaction for Photosynthesis is: Sunlight + 6CO2 + H2O = C6H12O6 + 6O2 9. Exothermic Reaction • Exothermic Chemical Reaction describes a chemical reaction that gives off heat energy. Example: mixture of sodium and chlorine to yield table salt. This reaction produces energy for each molecule of table salt that is produced. • Chemical Reaction for Sodium and Chlorine is: Na + Cl2 = NaCl + heat 10. Physical Change • The transformation of the external appearance of a substance that does not convert it into a new substance. 11. Precipitate • Precipitates can form when two soluble salts react in solution to form one or more insoluble products. The insoluble product separates from the liquid and is called a precipitate. • Precipitates can also form when the temperature of a solution is lowered. The low temperature reduces the solubility of a salt, resulting in its precipitation as a solid. 11-A. Precipitate • A precipitate is a solid that forms out of solution. A common example is that of the mixing of two clear solutions: (1) silver nitrate (AgNO3) and (2) sodium chloride (NaCl): The chemical reaction is: • The precipitate forms because the solid (AgCl) is insoluble in water. That is true for all precipitates - the solids are insoluble in aqueous solutions. 11-B. Precipitate • Precipitation reactions occur all around us. For example, sometimes the pipes in our homes get clogged because precipitates of magnesium and calcium oxides have deposited themselves within the pipes. This can happen with "hard" water. Another example is a kidney stone. A kidney stone is nothing more than a precipitate - often of calcium ions (from cheese) and oxalates. It is often suggested that a good way to avoid kidney stones is to drink a lot of water. This helps because the solubility of the precipitate increases with the amount of water - thus avoiding the formation of the kidney stone to begin with. 12. Reactants • The substances or compounds that you start with in a chemical equation (left side). • Substances initially present in a chemical reaction that are consumed during the reaction to make products. • 6CO2 + 6H2O + Sunlight C6H12O6 + 6O2 + Heat Reactants Products 12. Reactants • The substances or compounds that you start with in a chemical equation (left side). REACTANT 13. Products • A product is a substance that forms as a result of a chemical reaction. The end product of some chemical reactions may be the result of a relatively rapid reaction, nanoseconds to seconds. • The substances or compounds that you have at the end of a chemical equation (right side). • 6CO2 + 6H2O + Sunlight C6H12O6 + 6O2 + Heat Reactants Products 13. Products • The substances or compounds that you have at the end of a chemical equation (right side). PRODUCT 14. Subscript • Subscripts are placed to the lower right of the elements symbol to show the number of atoms of the element in a molecule. • Formulas for molecules use chemical symbols with subscript numbers to show the number of atoms of each element: • Examples: • O2 for molecular oxygen • O3 for ozone • CH4 for methane • C6H6 for benzene. • Parentheses may enclose atoms that act as a group.