Alchemy Unit Investigation II
advertisement

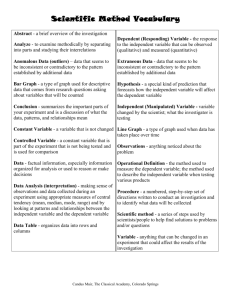
Alchemy Unit Investigation II: Basic Building Materials QuickTime™ and a QuickD raw decompressor are needed to see this picture. Lesson 1: A New Language Lesson 2: Now You See It . . . Lesson 3: What Goes Around Lesson 4: Create a Table Lesson 5: Breaking the Code Alchemy Unit – Investigation II Lesson 1: A New Language QuickTime™ and a QuickD raw decompressor are needed to see this picture. ChemCatalyst There are two bottles on a shelf in a chemistry lab. Both contain a shiny metal substance that resembles gold. Bottle A is labeled Au(s). Bottle B is labeled FeS2(s). • Do you think both bottles contain gold? Why or why not? • What do you think the symbols on the bottles mean? © 2004 Key Curriculum Press. Unit 1 • Investigation II The Big Question • What do the chemical symbols tells us about the substance inside the bottle? © 2004 Key Curriculum Press. Unit 1 • Investigation II You will be able to: • Make sense of chemical names and symbols. © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes • An element is a unique form of matter that serves as a building material for more complex matter. • Elements cannot be broken apart into two different substances. © 2004 Key Curriculum Press. Unit 1 • Investigation II Activity Purpose: The goal of this lesson is to give you practice making sense of some of the “language” of chemistry, and translating chemical names and formulas. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II Name Chemical formula Description Vial 1 Vial 2 copper nitrate Vial 3 blue-green crystals Vial 4 Vial 5 NaNO3(s) Vial 6 Vial 7 Vial 8 Vial 9 nitric acid © 2004 Key Curriculum Press. Unit 1 • Investigation II Name Chemical formula Vial 10 Description fine, brown powder Vial 11 NaOH(aq) Vial 12 Vial 13 Vial 14 Vial 15 clear liquid zinc sulfate Vial 16 Vial 17 Cu(NO3)2(aq) Vial 18 © 2004 Key Curriculum Press. Unit 1 • Investigation II Making Sense • When you turned the penny silver on the first day of class, you used zinc, Zn(s), and sodium hydroxide, NaOH(aq). Do you think the penny was coated with silver, Ag(s)? Explain your reasoning. © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes • A chemical formula is the set of symbols a chemist uses to represent a compound. Carbon dioxide is a compound. Its chemical formula is CO2. • A compound is a substance that consists of two or more elements chemically combined together. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes (cont.) • A substance is aqueous if it is dissolved in water. • The substance that is dissolved with water is called the solute. • The water is referred to as the solvent. © 2004 Key Curriculum Press. Unit 1 • Investigation II Check-In • Imagine you find a vial that is labeled Na2SO4(aq). What does the label tell you about what is in this flask? © 2004 Key Curriculum Press. Unit 1 • Investigation II Wrap-Up • Chemical symbols represent the elements that combine to form various substances. Each element has either a one or two letter symbol. The first letter is always capitalized, the second letter is always lower case. • The chemical formula of a substance tells us what elements are in it as well as the relative amounts of each element in that substance. © 2004 Key Curriculum Press. Unit 1 • Investigation II Alchemy Unit – Investigation II Lesson 2: Now You See It . . . QuickTime™ and a QuickD raw decompressor are needed to see this picture. ChemCatalyst • What is the starting ingredient you will be using in this lab? • Why must part of the lab be done in a fume hood? • When you filter, will you keep the solid or the liquid? © 2004 Key Curriculum Press. Unit 1 • Investigation II The Big Question • What happens to copper once it has been mixed with other substances? © 2004 Key Curriculum Press. Unit 1 • Investigation II You will be able to: • Follow a procedure for a series of lab experiments and make observations. © 2004 Key Curriculum Press. Unit 1 • Investigation II Activity Purpose: In this lesson you will complete a formal laboratory procedure. You will begin with copper powder and take it through a series of chemical steps, carefully observing and recording what happens at each stage. The goal is to figure out what happened to the original copper. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II (cont.) • You will not be allowed to begin the experiment until you can answer the questions about the lab correctly. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II (cont.) Setup and Safety Precautions: This lab is safe, as long as each step is done carefully and correctly. Here are some safety guidelines. • Everyone will wear safety goggles at all times. • Be very careful handling the nitric acid, as it will burn any exposed skin. If some gets on your skin, wash the area immediately with water and inform your teacher. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II (cont.) • As you add the nitric acid to the copper, nitrogen dioxide, NO2(g), a poisonous gas, will be produced. Be careful not to breathe this gas. Use the fume hood when you add the nitric acid to the copper. • Always be careful when heating chemicals— they will burn exposed skin more quickly when hot. When using the hot plate, make sure to set it at a medium setting (e.g. setting 4 out of 10). Be especially careful not to splash when stirring the chemicals. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II (cont.) • Sulfuric acid is an extremely strong acid, although you are using it here in a fairly dilute (unconcentrated) form. If you get it on your skin, wash it off with plenty of water, and inform your teacher. © 2004 Key Curriculum Press. Unit 1 • Investigation II (cont.) What you added or did What you observed Got copper powder from teacher © 2004 Key Curriculum Press. Unit 1 • Investigation II Making Sense Experimental stage Observations 1. Copper at the start Brownish, copper-colored, fine solid. 2. After adding nitric acid (HNO3) Turned sky blue – liquid. Brownish gas. 3. After adding water (H2O) Still a blue liquid. 4. After adding sodium hydroxide (NaOH) Clumpy dark blue. 5. After heating Turned black. Black solid in clear liquid.* 6. After adding sulfuric acid (H2SO4) Clear blue solution. 7. After adding zinc (Zn) Bubbled. Chunks of solid appeared. 8. Final Zinc turned black and disappeared. © 2004 Key Curriculum Press. Unit 1 • Investigation II Check-In • No Check-In © 2004 Key Curriculum Press. Unit 1 • Investigation II Wrap-Up • No Wrap-Up © 2004 Key Curriculum Press. Unit 1 • Investigation II Alchemy Unit – Investigation II Lesson 3: What Goes Around QuickTime™ and a QuickD raw decompressor are needed to see this picture. ChemCatalyst • What do you think happened to the copper powder in the copper cycle experiment when it was mixed with the nitric acid? © 2004 Key Curriculum Press. Unit 1 • Investigation II The Big Question • What happened to the copper? © 2004 Key Curriculum Press. Unit 1 • Investigation II You will be able to: • Use your observations to draw conclusions and summarize them in a lab report. © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes “Nitric acid is added to solid copper powder, resulting in a blue solution and a brown gas.” HNO3(aq) is added to Cu(s), resulting in and . (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes (cont.) HNO3(aq) is added to Cu(s), resulting in Cu(NO3)2(aq) and NO2(g). “Nitric acid is added to solid copper powder, resulting in a solution of copper nitrate and the release of nitrogen dioxide gas.” © 2004 Key Curriculum Press. Unit 1 • Investigation II Activity Purpose: The goal of this activity is to use chemical symbols to keep track of an element when it goes through various chemical transformations. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II (cont.) removes H2O (water) (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II What you did Got a sample of copper Added nitric acid Chemical added What you saw Write the chemical formula Your observations from the lab Cu(s) orangishbrown fine powder Write the chemical formula and name of the copper compound at each stage Where is the copper? Cu(s) solid copper powder The copper is in the beaker because the teacher put it there. HNO3(aq) Added sodium hydroxide Added heat (removes H2O) none Added sulfuric acid Added zinc Cu(s) solid copper powder © 2004 Key Curriculum Press. Unit 1 • Investigation II Making Sense • How would you describe what happened to the copper throughout this experiment? © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes (cont.) • An element is a unique form of matter that serves as a building material for more complex matter. • Elements cannot be broken apart into two different substances. • When elements are combined under ordinary conditions, the elements are not destroyed. © 2004 Key Curriculum Press. Unit 1 • Investigation II Check-In Sodium chloride, NaCl(aq), is added to silver nitrate, AgNO3(aq), resulting in NaNO3(aq) and a white solid. Identify the white solid from the list below. A) AgCl(s) B) AgCl(aq) C) AgNO3(s) D) NaCl(s) © 2004 Key Curriculum Press. Unit 1 • Investigation II Wrap-Up • Elemental copper can be transformed through chemical reactions, and then recovered again. • We can represent elements with symbols and keep track of them during chemical reactions. • Elements combine and recombine but they are not destroyed in chemical reactions. © 2004 Key Curriculum Press. Unit 1 • Investigation II Alchemy Unit – Investigation II Lesson 4: Create a Table QuickTime™ and a QuickD raw decompressor are needed to see this picture. ChemCatalyst In 1889 a Russian chemistry teacher created an organized table of the elements. At the time only 63 different elements were known. Below is a reproduction of that table. • What do you think the numbers represent? (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II © 2004 Key Curriculum Press. Unit 1 • Investigation II The Big Question • How did Mendeleyev organize the elements? © 2004 Key Curriculum Press. Unit 1 • Investigation II You will be able to: • Explain how the periodic table of elements is organized. © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes • Dimitri Mendeleyev is credited with organizing the elements into the first periodic table. • The main properties that Mendeleyev used to sort the elements were reactivity with one another and a number describing the atomic weight of each element. © 2004 Key Curriculum Press. Unit 1 • Investigation II Activity Purpose: The goal of this lesson is to acquaint you with Mendeleyev’s organization of the elements by allowing you to create your own table from the patterns you see in the elements. © 2004 Key Curriculum Press. Unit 1 • Investigation II Making Sense Below are five possible cards for the element germanium. Where does germanium belong in the table? Which card seems most accurate to you? What is your reasoning? (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II Germanium Germanium Germanium Ge 62.7 Ge 62.7 Ge 66.0 A B C Germanium Ge 72.6 Germanium Ge 72.6 (cont.) D E © 2004 Key Curriculum Press. Unit 1 • Investigation II (cont.) • What would you add to the three empty corners to complete the card? Germanium Ge (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II (cont.) Completed Table © 2004 Key Curriculum Press. Unit 1 • Investigation II Check-In • Which of the following elements would you find in the same group on the periodic table? Explain your thinking. Cadmium Cd Moderately soft, silvery solid, metal React very slowly with water Found in CdCl2 (s) Zinc Zn Moderately hard, silvery solid, metal Reacts very slowly with water Found in ZnCl2 (s) Iodine I Purple solid, nonmetal Reacts slowly with metals Found in ICl (s) Mercury Hg Silvery liquid, metal Does not react with water Found in HgCl2 (s) © 2004 Key Curriculum Press. Unit 1 • Investigation II Wrap-Up • Mendeleyev organized the periodic table based on the properties of the elements. • Mendeleyev’s arrangement of the elements helped to predict the existence of undiscovered elements. © 2004 Key Curriculum Press. Unit 1 • Investigation II Alchemy Unit – Investigation II Lesson 5: Breaking the Code QuickTime™ and a QuickD raw decompressor are needed to see this picture. ChemCatalyst • Where did Mendeleyev place copper, Cu, on the periodic table he created? (Note: The atomic weight of copper is 63.) • Where would you put copper, Cu, on your periodic table? Explain your thinking. © 2004 Key Curriculum Press. Unit 1 • Investigation II The Big Question • How can you predict properties of elements using a periodic table? © 2004 Key Curriculum Press. Unit 1 • Investigation II You will be able to: • Interpret some of the information given in the periodic table. © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes (cont.) (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes (cont.) (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes (cont.) • The elements in the middle of the table are referred to as the transition elements, or the transition metals. © 2004 Key Curriculum Press. Unit 1 • Investigation II Activity Purpose: This lesson will help to identify many of the patterns that are contained in the periodic table of the elements. (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II (cont.) © 2004 Key Curriculum Press. Unit 1 • Investigation II Making Sense • The elements copper and gold are both relatively unreactive. It is easy to bend and shape both metals. Both are used to make coins and jewelry. Is the similarity in their properties consistent with their locations on the periodic table? Explain why or why not. © 2004 Key Curriculum Press. Unit 1 • Investigation II Notes © 2004 Key Curriculum Press. Unit 1 • Investigation II Check-In • Use the cards for Cu, copper, and Au, gold, to describe all you can about the element silver, Ag. Copper shiny, yellow metal Cu 63.5 not very reactive Gold shiny, reddish metal Au 197.0 found in AuCl reacts slowly in air found in CuCl © 2004 Key Curriculum Press. Unit 1 • Investigation II Wrap-Up • Elements in each column of the periodic table have similar properties. • We can predict the characteristics of a missing element based on the qualities of the elements found adjacent to it in a periodic table. © 2004 Key Curriculum Press. Unit 1 • Investigation II