Introduction and Molar Balances - University of Illinois Urbana
advertisement
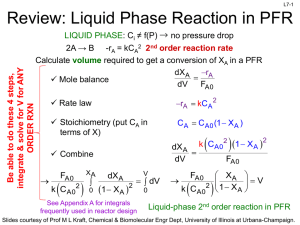
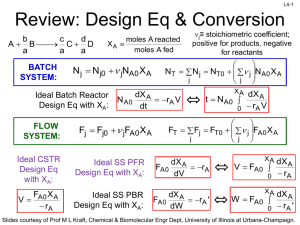
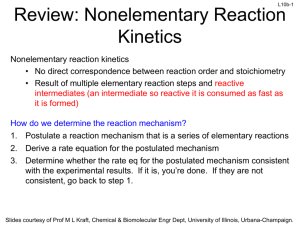
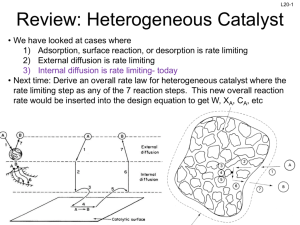
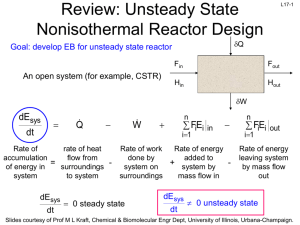
L1-1 CHBE 424: Chemical Reaction Engineering Introduction & Lecture 1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-2 What is Chemical Reaction Engineering (CRE) ? Understanding how chemical reactors work lies at the heart of almost every chemical processing operation. Raw material Separation Process Chemical process Separation Process Products By-products Design of the reactor is no routine matter, and many alternatives can be proposed for a process. Reactor design uses information, knowledge and experience from a variety of areas - thermodynamics, chemical kinetics, fluid mechanics, heat and mass transfer, and economics. CRE is the synthesis of all these factors with the aim of properly designing and understanding the chemical reactor. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-3 How do we design a chemical reactor? Type & size Maximize the space-time yield of the desired product (productivity lb/hr/ft3) Stoichiometry Kinetics Basic molar balances Fluid dynamics Reactor volume Use a lab-scale reactor to determine the kinetics! Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-4 Reactor Design Reaction Stoichiometry Kinetics: elementary vs non-elementary Single vs multiple reactions Reactor Isothermal vs non-isothermal Ideal vs nonideal Steady-state vs nonsteady-state Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-5 What type of reactor(s) to use? in Continuously Stirred Tank Reactor (CSTR) out Well-mixed batch reactor Plug flow reactor (PFR) Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-6 What size reactor(s) to use? Answers to this questions are based on the desired conversion, selectivity and kinetics Reactor type & size Kinetics Material & energy balances Conversion & selectivity Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-7 Chemical Reaction • A detectable number of molecules have lost their identity and assumed a new form by a change in the kind or number of atoms in the compound and/or by a change in the atoms’ configuration • Decomposition • Combination • Isomerization • Rate of reaction – How fast a number of moles of one chemical species are being consumed to form another chemical species Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-8 Rate Law for rj • rA: the rate of formation of species A per unit volume [e.g., mol/m3•s] • -rA: the rate of a consumption of species A per unit volume A B products r A kC A CB 1st order in A, 1st order in B, 2nd order overall r A kCAn rA nth order in A k1CA Michaelis-Menton: common in enzymatic reactions 1 k 2CA rj depends on concentration and temperature: -rA Ea RT C A e A Arrhenius dependence on temperature A: pre-exponential factor R : ideal gas constant E A : activation energy T:temperature Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-9 Basic Molar Balance (BMB) Fj0 Fj Gj System volume Rate of flow of j into system Rate of Rate of Rate of flow of j + generation of j Rate of decomposition = out of by chemical accumulation of j system rxn combine Fj 0 mol s in Fj mol s - out Gj mol s + generation Nj: moles j in system at time t dN j dt d mol dt = accumulation Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-10 Basic Molar Balance (BMB) Rate of flow of j into system Fj 0 mol s Rate of Rate of Rate of flow of j + generation of j Rate of decomposition = out of by chemical accumulation of j system rxn Fj mol s Gj dN j dt mol s d mol dt If the system is uniform throughout its entire volume, then: G j rj V Moles Moles j generated per generated = unit time and per unit time volume (mol/s) (mol/s•m3) Volume (m3) Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-11 Non-Uniform Generation system If rj varies with position (because the temperature or concentration varies) then rj1 at location 1 is surrounded by a small subvolume DV within which the rate is uniform DV V m G j lim DV Rate is rj1 within this volume rjDV rjdV Rate is rj2 within this volume m→∞ i1 DV→0 z 1 111 then G j rj x, y, z dx dy dz 000 1 1 y Plug in rj and integrate over x, y, and z x Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-12 Basic Molar Balance Equations Fj0 Fj Gj System volume In - Out + Generation = Accumulation dN j Fj0 F j G j dt dN j Fj0 F j rj V dt V dN j Fj0 F j rjdV dt uniform rate in V nonuniform rate in V Next time: Apply BME to ideal batch, CSTR, & PFR reactors Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-13 Review of Frequently Encountered Math Concepts Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-14 Basic Math Review 1 x ln x y y ln x n x n p x q ln a e a q p x x ln x ln y ln y ln x ln y ln xy Example: Problems that Contain Natural Logs Solve for X: a ln bt 1 ln x ln y b a x a b ln bt 1 ln x ln y ln bt 1 ln b y ln bt 1a b e x ln e y bt 1 a b x y y bt 1 a b x Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L1-15 Review of Basic Integration 1 x b 1 b n p x n x n1 For n≠1: n dx x dx x ax a b n q q p x b b n1 a n1 n 1 a n 1 n 1 1 b b dx ln x ln ln b ln a For n=1: n a a x a Solve for t: 5 c t 1 1 c t 0 1 ct 1 2 dt t 0 5 d d d0 x 1 1x 5 dx c 0.2 1 t d c 0.8 t d d 0.8 t c Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. Solve for c: L1-16 k k dc dc c dt 1 k t dt 1 k t c d d t c dc 1 k dt 0 1 kdt c c Do NOT move t or c outside of the integral 0 x x x dx From 1 ln 1 x Appendix A: 1 x 0 0 ε is a constant t 1 c k ln 1 k d t ln c c 0 kd 0 1 1 k ln 1 k d t ln 1 k d 0 ln c ln c 0 kd 0 kd k ln k d t 1 ln c ln c 0 kd k ln k t 1 d kd e c ln c e 0 c k ln k d t 1 ln kd c0 k ln k dt 1 k e d c c0 c0 k ln k t 1 d kd e c Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.