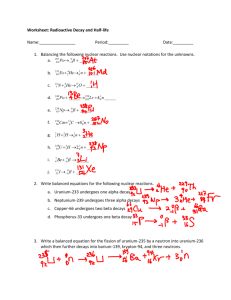
Name: Date: Period:____ Nuclear Chemistry Study Guide 1
advertisement
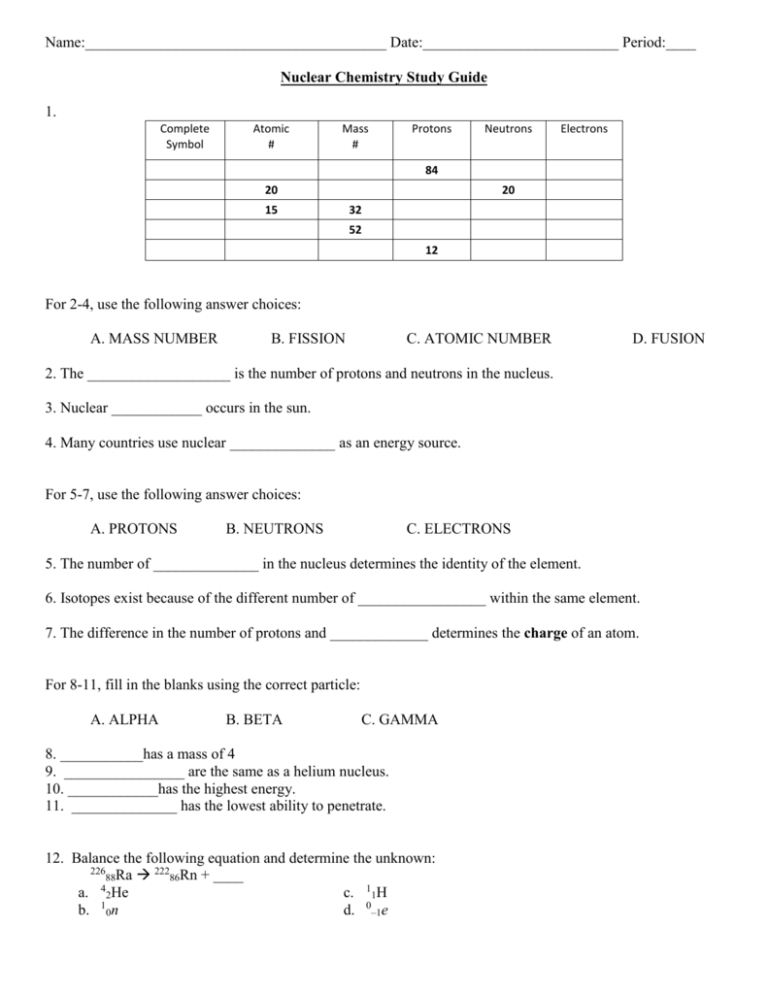
Name:________________________________________ Date:__________________________ Period:____ Nuclear Chemistry Study Guide 1. Complete Symbol Atomic # Mass # Protons Neutrons Electrons 84 20 15 20 32 52 12 For 2-4, use the following answer choices: A. MASS NUMBER B. FISSION C. ATOMIC NUMBER D. FUSION 2. The ___________________ is the number of protons and neutrons in the nucleus. 3. Nuclear ____________ occurs in the sun. 4. Many countries use nuclear ______________ as an energy source. For 5-7, use the following answer choices: A. PROTONS B. NEUTRONS C. ELECTRONS 5. The number of ______________ in the nucleus determines the identity of the element. 6. Isotopes exist because of the different number of _________________ within the same element. 7. The difference in the number of protons and _____________ determines the charge of an atom. For 8-11, fill in the blanks using the correct particle: A. ALPHA B. BETA C. GAMMA 8. ___________has a mass of 4 9. ________________ are the same as a helium nucleus. 10. ____________has the highest energy. 11. ______________ has the lowest ability to penetrate. 12. Balance the following equation and determine the unknown: 226 222 88Ra 86Rn + ____ 4 a. 2He c. 11H b. 10n d. 0–1e 13. Which type of radiation has no mass and no charge? a. 0-1β b. alpha particles c. 42He d. gamma rays 14. The half-life of an isotope is the time required for half the nuclei in a sample to a. undergo radioactive decay. c. undergo nuclear fusion. b. undergo nuclear fission. d. react chemically. 15. An isotope has a half-life of 25 days. If you start with 50 grams, how much of the substance will remain after 75 days? a. 25 grams c. 50 grams b. 12.5 grams d. 6.25 grams 16. Which type of reaction produces the most energy? a. fission c. chemical reaction of natural gas burning b. coal burning d. fusion 17. Beta particles have a _____ charge. a. -1 b. 0 c. +1 d. +2 18. What is the product of decay from a radioactive isotope of lead? a. Thallium c. Bismuth b. Mercury d. Lead 19. How long does it take a 100.00g sample of As-81 to decay to 6.25g? The half-life of As-81 is 33 seconds. 20. Cobalt-60 is a radioactive element used as a source of radiation in the treatment of cancer. Cobalt-60 has a half-life of five years. If a hospital starts with a 1000-mg supply, how much will remain after 10 years? a. 750 mg b. 500 mg c. 250 mg d.1000 mg Identify the following as alpha or beta decay: 21. 22. _________________________ ____________________________ Fill in the missing blanks on these reactions: 23. 24. 25. Complete the following table: Half-life 0 1 2 3 4 Years 0 5 Mass of Sample 200 26. The half-life of carbon-14 is 5730 years. If a 10 gram sample undergoes decay for 17,190 years, how many grams remain? 27. The half-life of lead-210 is 22 years. How many years does it take for a 1600 gram sample of lead-210 to be reduced to 100 g? Write a balanced equation for each of the following nuclear reactions: 28. Curium-240 (Cm) decays by alpha emission. 29. Americium-243 (Am) decays by alpha emission. 30. Krypton-87 (Kr) decays by beta emission. 31. Magnesium-27 (Mg) decays by beta emission. 32. Type of Radiation Alpha Beta Gamma Symbol Charge Mass