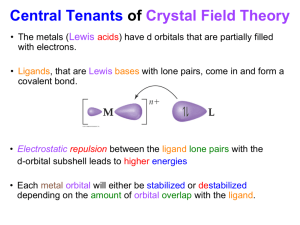
1 + -1
advertisement

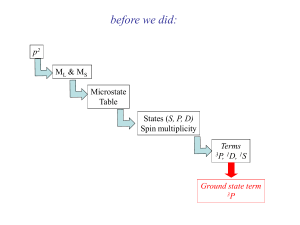
Electronic (UV-visible) Spectroscopy | Electronic | XPS UPS UV-visible 1 UV-visible spectroscopy ligand p* (1) metal-metal (d-d) transition (2) charge transfer s* metal d metal-ligand (MLCT) ligand-metal (LMCT) n metal d n ligand p (3) ligand-centered transition s instrument energy source sample energy selector energy analyzer output computer electric connection light path absorbance Io A = log ―― = ecl I e: extinction coefficient c: concentration mol/L (M) l: path length (cm) 2 selection rules 1. only one electron is involved in any transition 2. there must be no net change of spin DS = 0 3. it must involve an overall change in orbital angular momentum of one unit DL = ±1 4. Laporte (or parity) selection rule only g →u and u →g transitions are allowed vibronic coupling – interaction between electronic and vibrational modes electronic transition Laporte allowed (charge transfer) e 10000 (1000—50000) Laporte forbidden (d-d transition) spin allowed; noncentrosymmetiric 100—200 (200—250) spin allowed; centrosymmetric 5—100 (20—100) spin forbidden 0.01—1 (< 1) 3 [CoCl4]2- [Co(H2O)6]2+ [Mn(H2O)6]2+ 4 d-d transition crystal field splitting Do size and charge of the metal ion and ligands 4d metal ~50% larger than 3d metal 5d metal ~25% larger than 4d metal 5d > 4d > 3d crystal field stabilization energy (CFSE) spin-pairing energy high-spin/low spin configuration d4 ~ d7 d4 5 other shapes tetrahedral Dt = 4/9 Do tetrahedron octahedron elongated square octahedron planar 6 d1 [Ti(H2O)6]3+ hu = D o hole formalism d2 possible electron possible arrangements of electrons transitions 7 Russell-Saunders term symbols 2S+1L for free atoms and ions J S: total spin quantum number Sms L: total orbital angular quantum number Sml L = 0, 1, 2, 3, 4, ………….. S P D F G 1 3 5 7 9 J: total angular quantum number L+S, ……,│L-S│ d2 configuration S 10! ———— = 45 microstates 8! 2! +1 0 -1 L (2+ 2-) 4 (2+ 1+) (2+ 1-) (2- 1+) (2- 1-) 2 (2+ 0+) (1+ 1-) (2+ 0-) (2- 0+) (2- 0-) 1 (1+ 0+) (2+ -1+) (1+ 0-) (1- 0+) (2+ -1-) (2- -1+) (1- 0-) (2- -1-) (1+ -1+) (2+ -2+) (0+ 0-) (1+ -1-) (1- -1+) (2+ -2-) (2- -2+) (1- -1-) (2- -2-) 3 0 1G 9 3F 1D + 21 + 5 ground term 3P + 9 1S + 1 = 45 8 states for dn systems in Russell-Saunders coupling splitting of terms in various chemical environments d orbitals in Oh environment consider pure rotational O subgroup rotation by angle a ==> R(r), Q(q), ψs invariant only F(f) will be altered F(f) = eimf ==> F(f + a) = eim(f + a) m = 2, 1, 0, -1, -2 e2if e2i(f + a) eif ei(f + a) e0 ======> e0 e-if e-i(f + a) 9 e-2if e-2i(f + a) transformation matrix e2ia 0 0 0 0 ei a 0 0 0 0 e0 0 0 0 0 e-ia 0 0 0 0 sum of the diagonal elements sin(l + 1/2)a c (a) = ——————— sin(a/2) 0 0 0 0 e-2ia for d orbitals c (E ) = 5 sin(5p/2) c (C2) = ————— = 1 sin(p/2) sin(5p/3) sin(5p/4) c (C3) = ————— = -1 c (C4) = ————— = -1 sin(p/3) sin(p/4) ==> G = eg + t2g 10 splitting of one-electron levels in an Oh environment splitting of one-electron levels in various symmetries 11 determine the spin multiplicity of each term d2 configuration in Oh environment aA + bE + cT + dT (i) t2g2 1g g 1g 2g total degeneracy 15 a b c I 1 1 1 II 1 1 3 III 3 3 1 d 3 1 1 a T + bT (ii) t2g1eg1 1g 2g total degeneracy 24 only possibility 1T1g 1T2g 3T1g 3T2g aA + bA + cE (iii) eg2 1g 2g g total degeneracy 6 a b c I 1 3 1 II 3 1 1 1S 1A 1g 1G 1A 1g 3P 3T 1g 1 E 1T g 2g 3A 3 3 1g T1g T2g 1D 3F 1E 1T 1 g 1g T2g 12 method of descending symmetry consider d2 ion in Oh environment from correlation table for group Oh (i) t2g2 A1g Eg T1g T2g lowering the symmetry to C2h 1A 1E t2g × t2g = 1g g possible spin 1 1 multiplicity 1 1 3 3 1A corresponding 1Ag g 1B representations g in C2h ag × ag Ag ====> ag × ag’ Ag ====> a g × bg Bg ====> ag’ × ag’ Ag ====> a g ’ × bg Bg ====> bg × bg Ag ====> t2g 3T 1g 1 3 1 3A g 3B g 3B g 1A g 1A 3A g g 1B 3B g g 1A g 1B 3B g g 1A g ===> total 41Ag + 3Ag + 21Bg + 23Bg a g + a g + bg 1T 2g 3 1 ˇ 1 1A g 1A g 1B g 13 (ii) eg2 A1g A2g Eg lowering the symmetry to D4h eg a1g + b1g a1g2 A1g possible spin multiplicity 1A1g a1gb1g B1g possible spin multiplicity 1B1g 3B1g b1g2 A1g possible spin multiplicity 1A1g ==> D4h Oh 1A 1A 1g 1g 3B 3A 2g 1g 1 A 1B 1E 1g 1g g (iii) t2g1eg1 ???? consider d2 ion in Td environment from splitting of energy level in Td symmetry 3F 3A 3T 3T 2 1 2 1D 1E 1T 2 3P 3T 1 1G 1A 1E 1T 1 T 1 1 2 1S 1A 1 electron configurations e2 A1 A2 E total degeneracy 6 et2 T1 T2 total degeneracy 24 t22 A1 E T1 T2 total degeneracy 15 assign the correct spin multiplicity ??? 14 splitting of the terms for d2 ion in several point groups 15 correlation diagram for a d2 ion in Oh environment 16 correlation diagram for a d2 ion in Td environment 17 Orgel diagrams d1, d6/d4, d9 d1, d6 tetrahedral d4, d9 octahedral d1, d6 octahedral d4, d9 tetrahedral u = 10 Dq E Eg T2 T2 g T2g T2 Eg E 18 d2, d7/d3, d8 cm-1 d2, d7 tetrahedral d3, d8 octahedral Dq d2, d7 octahedral d3, d8 tetrahedral A2→T2 u1 = 10Dq T1→T2 u1 = 8Dq + c A2→T1(F) u2 = 18Dq - c T1(F)→T1(P) u2 = 18Dq + c A2→T1(P) u1 = 15B + 12Dq + c T1→A2 u3 = 15B + 6Dq + 2c 19 20 Tanabe-Sugano diagrams 21 22 simplified Tanabe-Sugano diagrams d2 d5 d3 d6 d4 d7 d8 23 magnitude of Do Mn(II) < Ni(II) <Co(II) < Fe(II) < V(II) < Fe(III) < Cr(III) < V(III) < Co(III) < Mn(IV) < Mo(III) < Rh(III) < Pd(IV) < Ir(III) < Re(IV) < Pt(IV) Do values for octahedral [M(H2O)6]n+ complexes Do (cm-1) Ti3+ 20400 Mn3+ 21000 Co3+ 19000 V3+ 19000 Mn2+ 7500 Co2+ 9750 Cr3+ 17700 Fe3+ 21000 Ni2+ 8500 Cr2+ 12500 Fe2+ 10500 Cu2+ 12600 spectrochemical series I- < Br- < -SCN- < Cl- < F- < urea < OH- < CH3COO< C2O4- < H2O < -NCS- < glycine < pyridine ~ NH3 < en < SO32- < o-phenanthroline < NO2- < CN- < PR3 < CO ex. [Co(H2O)6]3+ Do = 19000 cm-1 [Co(NH3)6]3+ Do = 22900 cm-1 [Co(H2O)3(NH3)3]3+ Do = ? 3/6 × 19000 + 3/6 × 22900 = 20950 cm-1 24 Jørgensen prediction of 10Dq and B 10Dq = f · g (cm-1 × 10-3) B = Bo (1 - h · k) Bo : free ion interelectronic repulsion parameter Jahn-Teller distortions distortion will occur whenever the resulting splitting energy levels yields additional stabilization __ dx2-y2 __ dz2 eg __ __ __ dz2 __ dx2-y2 or __ dxy __ __dxz, dyz t2g __ __ __ __ __ dxz, dyz __ dxy 25 [M(H2O)6]n+ Ti3+ (d1) Mn2+ (d5) V3+ (d2) Fe2+ (d6) Cr3+ Co2+ (d7) (d3) Ni2+ (d8) Cu2+ (d9) Cr2+ (d4) 26 d1 d2 27 d3 28 d3 29 d4 d5 30 d6 31 d6 32 d6 33 d7 34 d8 d9 35







![Electronic Transitions - Department of Chemistry [FSU]](http://s3.studylib.net/store/data/009485495_1-1bfd4c306334ada75c4b7fed912f73bd-300x300.png)