v - Piri Reis Üniversitesi
advertisement
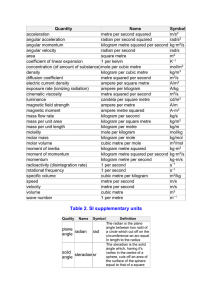
Physics -I Piri Reis University 2010-2011 Purpose of Course Introductory Physics o o o o o Physics, Engineering, Materials Science are all closely connected. Physical principles underlie many disciplines including engineering. Good understanding of the physics can guide best technological practice. Engineering is the application of physics to real situations. Composite materials (new types of materials) rely in some cases on physics. Of course there is much more to engineering than physics, but many aspects of engineering benefit from a good understanding of principles. Purpose of Course At the end of the course students should have a basic knowledge of: Mechanics; Fluid dynamics; Waves There is a heavy emphasis on Mechanics, which will include o Statics – balance of forces and torques o Dynamics – mechanics of motion o Energy, momentum, angular momentum o Mass, moment of inertia, o Newton’s Laws Structure of Course There are 14 weeks, each with 3 hours of theory and 2 hours dedicated to laboratory exercises and problem solving Students should: o Attend the lectures and practical classes. o Solve the set problems. o Hand in homework when requested. Lectures are more useful if you read ahead o Lecture content by week is outlined [approximate] o Read about subject before lecture [see reference list] o Ask questions in class Week Structure of Course 1 Introduction Basics: units, numbers, dimensional analysis, maths reminders 2 1-D Kinematics Speed, velocity, acceleration, Reference frames 3 2-D Kinematics Vectors, 2-D kinematics, projectile motion 4 Newton’s Laws Newton’s Laws and Gravity. 5 Linear Dynamics Mass, Weight, Force, Friction. Application of Newton’s Laws. 6 Linear Dynamics Work, energy, power. Conservation Laws 7 Linear Dynamics Momentum and motion of systems. Collisions. 8 Rotational Dynamics Rotation and Angular Momentum 9 Equilibrium Mechanics Static equilibrium of rigid bodies 10 Fluids Density, Buoyancy force, Archimedes' Principle 11 Thermodynamics Heat, temperature, the expansion of solids and gases, Gases, Heat transfer. 12 Materials The physical phase changes. Vapours, cooling. 13 SHM & Waves Simple harmonic motion and waves. 14 Travelling Waves Sound, Light Structure of Course Examinations o There will be two mid-term exams and o One final examination. o The examinations will be at the same level as the homework problems BASICS Units o There are two kinds o Base units (there are seven of these in any system) these cannot be defined in terms of anything else o Derived units With a special name With a compound name o Which are base and which derived is a choice - ‘common sense’ o In this course the base units mostly used are o Time in seconds, s o Mass in kilograms, kg o Length in metres, m [but really this is derived from c] o A numerical quantity without its unit is meaningless BASICS Some Derived Units o Motion o speed m/s o acceleration m/s2 o Energy o Energy is measured in Joules, J but [J] = [kg][m2]/[s2] o Momentum has no separate name and is [kg][m]/[s] o Angular speed is in [radians]/[s] This is a special one as radians have no unit - why o A numerical quantity without its unit is meaningless Name BASICS m length kilogram kg mass second s time ampere A electric current kelvin K thermodynamic temperature candela cd luminous intensity mol amount of substance mole hertz Hz frequency 1/s s-1 radian rad angle m·m-1 dimensionless steradian sr solid angle m2·m-2 dimensionless kg·m·s−2 newton N force, weight kg·m/s2 pascal Pa pressure, stress N/m2 m−1·kg·s−2 joule J energy, work, heat N·m = C·V = W·s m2·kg·s−2 watt W power, radiant flux J/s = V·A m2·kg·s−3 coulomb C electric charge s·A s·A volt V voltage W/A = J/C m2·kg·s−3·A−1 farad F electric capacitance C/V m−2·kg−1·s4·A2 ohm Ω electric resistance V/A m2·kg·s−3·A−2 siemens S electrical conductance 1/Ω m−2·kg−1·s3·A2 weber Wb magnetic flux J/A m2·kg·s−2·A−1 tesla T magnetic field strength V·s/m2 = Wb/m2 = N/(A·m ) kg·s−2·A−1 henry H inductance V·s/A = Wb/A m2·kg·s−2·A−2 Celsius °C temperature K − 273.15 K − 273.15 cd·sr lumen lm luminous flux lux lx illuminance lm/m2 m−2·cd·sr becquerel Bq radioactivity 1/s s−1 gray Gy absorbed dose J/kg m2·s−2 sievert Sv equivalent dose J/kg m2·s−2 −1 Quantity metre SI system of units – this is the usual choice lx·m2 Unit symbol Base units Derived units (with a special name) Compound units derived from SI units Expression in terms Name BASICS Some of the derived SI units with no special name Symbol Quantity of SI base units square metre m2 area m2 cubic metre m3 volume m3 metre per second m/s speed, velocity m·s−1 cubic metre per second m3/s volumetric flow m3·s−1 metre per second squared m/s2 acceleration m·s−2 metre per second cubed m/s3 jerk, jolt m·s−3 metre per quartic second m/s4 snap, jounce m·s−4 radian per second rad/s angular velocity s−1 newton second N·s momentum, impulse m·kg·s−1 newton metre second N·m·s angular momentum m2·kg·s−1 newton metre N·m = J/rad torque, moment of force m2·kg·s−2 newton per second N/s yank m·kg·s−3 reciprocal metre m−1 wavenumber m−1 kilogram per square metre kg/m2 area density m−2·kg kilogram per cubic metre kg/m3 density, mass density m−3·kg cubic metre per kilogram m3/kg specific volume m3·kg−1 mole per cubic metre mol/m3 amount (-of-substance) m−3·mol cubic metre per mole m3/mol molar volume m3·mol−1 joule second J·s action m2·kg·s−1 joule per kelvin J/K heat capacity, entropy m2·kg·s−2·K−1 joule per kelvin mole J/(K·mol) molar heat capacity m2·kg·s−2·K−1·mol−1 joule per kilogram kelvin J/(K·kg) specific heat capacity m2·s−2·K−1 joule per mole J/mol molar energy m2·kg·s−2·mol−1 joule per kilogram J/kg specific energy m2·s−2 joule per cubic metre J/m3 energy density m−1·kg·s−2 newton per metre N/m = J/m2 surface tension kg·s−2 watt per square metre W/m2 heat flux density, irradiance kg·s−3 watt per metre kelvin W/(m·K) thermal conductivity m·kg·s−3·K−1 SI system is not the only unit system. There are several. In nautical environment for example, the standard unit of speed for sailing is knots. 1 international knot = 1 nautical mile per hour (by definition), 1.852 kilometres per hour (exactly), 0.514 metres per second, 1.151 miles per hour (approximately). To convert 12mph to knots, divide by 1.151 12mph = 10.43 knots BASICS Writing Numbers o There is more than one way to write an answer o 0.000001 is equivalent to 1 x 10-6 which is also 1m In scientific notation write one figure before decimal point 1.23 x 104 NOT 12.3 x 103 o Precision is a measure of repeatability, a number can be very precise but the wrong answer. A precise measurement is one that always gives the same answer. o Accuracy is a measure of how close to the correct value the number is. An accurate measurement is one which is close to the real value. o The number of decimal places used in a number should reflect its accuracy (usually). If not then the precision and the accuracy need to be specified explicitly. BASICS Accuracy and Conversion o Writing the answer to a computation as 1.76 implies that the answer is accurate to 0.005 which would be quite accurate. The answer is between 1.755 and 1.765 o Round answers to reflect the actual precision in a calculation. o eg if you use g=9.81 m/s2 then answers are only accurate to 2 decimal places. So don’t list any extra useless decimal places. o The overall accuracy of a calculation is limited by the least accurate number in the calculation. o It does no good to use a very accurate constant with an inaccurate measurement. Round off numbers eg don’t use p = 3.141592654 if you are using g=9.81 o Approximations – use approximate numbers when appropriate BASICS Prefix Symbo l Decim al yotta Y 1E+24 zetta Z 1E+21 exa E 1E+18 peta P 1E+15 tera T 1E+12 giga G 1E+09 mega M 100000 0 kilo k 1000 hecto h 100 deca da 10 Prefixes and Suffixes o Base units are defined to be ‘sensible’ for humans. One kilogram of meat can be eaten. One metre can be stepped over One second can be counted o But these are not always a useful size in physics o The units are frequently used with letters to indicate a change in size Normally we only use these 1 deci d 0.1 centi c 0.01 milli m 0.001 micro μ 0.0000 01 nano n 1E-09 pico p 1E-12 femto f 1E-15 atto a 1E-18 zepto z 1E-21 yocto y 1E-24 BASICS Prefixes and Suffixes o o o o o 1000 m = 1 km 1000 byte = 1 kbyte 1000,000,000 = 1 Gbyte 1/1000 m = 1 mm 1/1000,000 m = 1 mm o note well: m = milli 10-3 M = mega 106 despite newspapers getting it wrong! o There is one other commonly used prefix, which is not SI o The Angstrom, Å, 10-10 m (about the size of an atom) BASICS Dimensional Analysis o It is critical to efficient problem solving that dimensional analysis is used After ‘every’ step in a calculation Certainly at the end of a calculation To help figure out what the correct formulae is ! o It is not possible to equate apples and oranges. o It makes no sense to write down a length in [kg] o A formula must be dimensionally consistent on the two sides o ie Units must match – apples and oranges cannot be equated o Dimensional analysis is the business of matching units (dimensions) + = 2 BASICS Dimensional Analysis For example, suppose we want to know the formulae for the volume of a sphere. It must depend on radius, but in what way? o Volume, V, has dimensions [m3] o There are no other variables in the problem except radius. o Radius, R, has the dimensions [m] o Therefore V = k R3 where k is a constant we don’t know. [its 4p/3] o This was a ‘simple’ example, but it works for more complex cases o [in fact its been used to discover ‘unknown’ equations!] + = + BASICS Notation Notation is key to communicating. its important to use the same variables as everyone else. o Symbol choice helps clarify equations o A famous formula is V = I R where V is voltage, I current and R resistance. o It would be confusing to write R = V I where R is voltage, V the current and I the resistance [ but it is not actually wrong] o Learn the conventional (standard) notation and use it. o m – mass; v – velocity; a – acceleration; t – time; I – moment of inertia; w – angular velocity; o note that vector quantities are underlined often [see later] o Some quantities, such as moment of inertia, need tensors, in this course we will use simple scalars to describe them. Angles and Angular Quantities o Usually use Greek letters for angles o a b c …. Vector & Scalar Notation o Vectors may be written as v or v or v It is usual to use an underline in hand written versions And bold in printed – The book by Giancoli uses v which is unusual o Scalar quantities are simply written as s Review of some Mathematics Linear Algebra Manipulation of linear equations If Then A+B=C B=C–A If Then AB = C B = C/A If Then A(B+C) = D AB + AC = D AB = BC ; A+B=B+A Associative Commutative Distributive Review of some Mathematics Linear Algebra Manipulation of linear equations If And Ax + By = C Dx - Ey = B Then x = DB/E + C – FB/E y = EA/B + F – CA/B Simultaneous equations - DO not use these formulae! Review of some Mathematics Trigonometry For a right angled triangle C2 = B2 + A2 Pythagoras’ theorem Sine(a) = A/C Cosine(a) = B/C Tangent(a) = A/B Use sin(a) cos(a) & tan(α) b C A a 90º B Review of some Mathematics (cosine,sine) Sines & Cosines around a circle In radians and degrees Review of some Mathematics Trigonometry There are many identities, these are particularly useful: Review of some Mathematics Calculus o Differentiation – •the rate of change •the instantaneous slope •the limit of Dy/Dx As Dx –> 0 m= m = positive m = zero m = negative Review of some Mathematics Calculus o Differentiation – o Some basic derivatives o dyn/dx = yn-1 o dcos(a)/da = sin(a) o dex/dx = ex o tables of these exist Review of some Mathematics Calculus o Integration – •the opposite of differentiation •the area under a curve = o Indefinite Integral If Then = F(x) = F(b) – F(a) Review of some Mathematics Calculus o Integration – o Some basic indefinite integrals