File - Honors Chemistry
advertisement

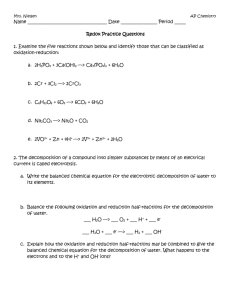
Solution Chemistry Net Ionics And RedOx Reactions Major Difference: • soluble ionic compounds exist as IONS Three Types of Chemical Reactions • Complete Chemical Reaction • Complete Ionic Reaction • Net Ionic Reaction Complete Chemical Reaction: • shows the chemical process in the non-ionized form • uses parenthetical notation to indicate the states of matter Examples 1) 2 NaOH (aq) + CuCl2 (aq) 2 NaCl (aq) + Cu(OH)2 (s) 2) HBr (aq) + NaOH (aq) NaBr (aq) + H2O (l) (acid) (base) 3) CaCO3 (s) + (carbonate) 2 HCl (aq) CO2 (g) + H2O (l) + CaCl2 (aq) (acid) Complete Ionic Reaction: • shows aqueous solutions in the ionic form (THEY ARE BROKEN APART) ▫ Coefficients are distributed to each ion ▫ Ion subscripts become coefficients • non-soluble substance, non-ionic substances, gases, liquids and precipitates are written in standard form (THEY ARE KEPT TOGETHER) • Ex: ▫ 2 NaOH (aq) 2 Na+ + 2 OH▫ CuCl2 (aq) Cu+2 + 2 Cl▫ H2O (l) No change ▫ CO2 (g) No change ▫ CaCO3 (s) No Change Not Aqueous 1) 2 NaOH (aq) + CuCl2 (aq) 2 NaCl (aq) + Cu(OH)2 (s) Becomes: 1) 2 Na+ + 2 OH- 1) 2 NaOH (aq) + CuCl2 (aq) 2 NaCl (aq) + Cu(OH)2 (s) Becomes: 1) 2 Na+ + 2 OH- + Cu2+ + 2 Cl- 1) 2 NaOH (aq) + CuCl2 (aq) 2 NaCl (aq) + Cu(OH)2 (s) Becomes: 1) 2 Na+ Cl- + 2 OH- + Cu2+ + 2 Cl- 2 Na+ + 2 1) 2 NaOH (aq) + CuCl2 (aq) 2 NaCl (aq) + Cu(OH)2 (s) Becomes: 1) 2 Na+ + 2 OH- + Cu2+ + 2 Cl- 2 Na+ + 2 Cl- + Cu(OH)2 2) HBr (aq) + NaOH (aq) NaBr (aq) + H2O (l) Becomes 2) H+ + Br-+ Na+ + OH- Na+ + Br- + H2O 3) CaCO3 (s) + 2 HCl (aq) CO2 (g) + H2O (l) + CaCl2 (aq) Becomes: 3) CaCO3 + 2 H+ + 2 Cl- CO2 + H2O + Ca+2 + 2 Cl- Net Ionic Reactions • shows the parts of the reaction that actually undergo change • ignores ions that remain unchanged on both sides of the reaction • called SPECTATOR IONS because they are not part of the process • the most simplified equation Examples 1) 2 Na+ + 2 OH- + Cu2+ + 2 Cl- 2 Na+ + 2 Cl- + Cu(OH)2 Examples 1) 2 Na+ + 2 OH- + Cu2+ + 2 Cl- 2 Na+ + 2 Cl- + Cu(OH)2 Examples Net Ionic: 2 OH- + Cu2+ Cu(OH)2 2) H+ + Br-+ Na+ + OH- Na+ + Br- + H2O 2) H+ + Br-+ Na+ + OH- Na+ + Br- + H2O Net Ionic: • H+ + OH- H2O 3) CaCO3 + 2 H+ + 2 Cl- CO2 + H2O + Ca+2 + 2 Cl- 3) CaCO3 + 2 H+ + 2 Cl- CO2 + H2O + Ca+2 + 2 Cl- Net Ionic: CaCO3 + 2 H+ CO2 + H2O + Ca+2 REDUCTION and OXIDATION REACTIONS Chemical reactions that show a change of charge in the atoms of the substances. Reduction: substances that gain electrons (charge is reduced) Oxidation: substances that lose electrons (charge is increased) • LEO goes GER • Loss of Electrons equals Oxidation • Gain of Electrons equals Reduction • Oxidizing Agent: the substance that removes electrons (gets reduced) • Reducing Agent: the substance that gives away electrons (gets oxidized) Examples • 2 K + Br2 2 KBr ▫ ▫ ▫ ▫ ▫ Initial charge of K and Br2 is neutral K becomes K+1 and Br becomes Br-1 K lost electrons to Br K is oxidized (reducing agent) Br is reduced (oxidizing agent) • N2 + 3 H2 2 NH3 N2 = no charge H2 = no charge NH3 = N-3 and H+1 Oxidizing agent = N Reducing agent = H • 2 Al + 6 HCl 2 AlCl3 + 3 H2 Reactant Side: Al = no charge H = +1 Cl = -1 Oxidized = Al Reduced = H Product Side: Al = +3 H = no charge Cl = -1 Determining Oxidation Numbers 1. Free elements are assigned an oxidation state of zero. • Ex. Metals (K, Fe, Al), Diatomics 2. The sum of the oxidation states of all that atoms in a species must be equal to the net charge on the species. • Ex. ClO3- Cl+5 + (O-2)3 = -1 3. The alkali metals (Li, Na, K, Rb, and Cs) in compounds are always assigned an oxidation state of +1. • Ex. KCl, HBr 4. Fluorine in compounds is always assigned an oxidation state of -1. • Ex. HF 5. The alkaline earth metals (Be, Mg, Ca, Sr, Ba, and Ra) and also Zn and Cd in compounds are always assigned an oxidation state of +2. • Ex. CaO 6. Hydrogen in compounds with other non-metals that are more electronegative is assigned an oxidation state of +1. • Ex. HCl, NH3 7. Hydrogen in compounds with metals is assigned an oxidation number of -1. • Ex. KH 8. Oxygen in compounds is assigned an oxidation state of -2. • Ex. H2O, CaO 9. Halogen in compounds is assigned an oxidation state of -1. • Ex. HF Examples 1) PO4 -3 2) CaF2 3) Zn 4) CH4 5) ClO4-1 6) ClO2-1 7) O2 8) N2O3 • What is an activity series, and how is it used? • From: http://antoine.frostburg.edu/chem/senese/101/redox/faq/activity-series.shtml • An activity series is a list of substances ranked in order of relative reactivity. For example, magnesium metal can knock hydrogen ions out of solution, so it is considered more reactive than elemental hydrogen: • Mg(s) + 2 H+(aq) H2(g) + Mg2+(aq) • Zinc can also displace hydrogen ions from solution: • Zn(s) + 2 H+(aq) H2(g) + Zn2+(aq) • so zinc is also more active than hydrogen. But magnesium metal can remove zinc ions from solution: • Mg(s) + Zn2+(aq) Zn(s) + Mg2+(aq) The most active metals are at the top of the table; the least active are at the bottom. • For a reaction to occur the more active element will reduce the other element. This usually occurs when the more active element is in the neutral (elemental) state and the less reactive element is an ion. • Each metal is able to displace the elements below it from solution • OR, each metal can reduce the cations of metals below it to their elemental forms. • Predicting Products Using the Activity Series: Ex: zinc metal in a copper(II) sulfate solution copper placed into a zinc sulfate solution