Ionic bonds and some main
advertisement

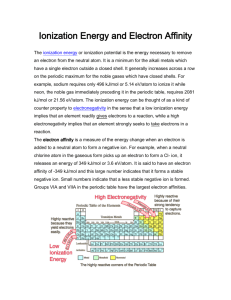
Ionic bonds and some maingroup chemistry Chapter 6 Ions and their electron configurations • Metals – tend to give up electrons, coming from the highest-energy occupied orbital. Sodium atom (1s22s22p63s1) reacts with chlorine and gives up an electron from the 3s, giving an ion with noble gas electron configuration of neon (1s22s22p6) • Nonmetals – tend to accept electrons, fill vacancy in 3p subshell to give electron configuration of argon (1s22s22p63s23p6) Problem: Ion configuration Write the ground state configuration for Mg. Write the ion configuration for Mg. Write the ground state configuration for O. Write the ion configuration for O. Transitional metal ions • As usual the transitional metals are different. They react with nonmetals to lose their 4s orbital 1st and then their 3d electrons. Ion formation is different than the building up process, so can’t be “reversed.” In building up also adding a proton / positive charge to nucleus. Ionic radii • Cation radii are smaller than their neutral atoms, both because their principal quantum number of the valence – shell is smaller for cations and because Zeff (effective nuclear charge) is larger from decrease in electrons. • Anions radii are larger than their neutral atoms because of additional electron-electron repulsions and a decrease of Zeff from the additional electrons. Problem: Radii comparison • Which atom or ion in each of the following pairs would you expect to be larger? • O or O-2 • O or S • Fe or Fe+3 Ionizing energy • Ei is the amount of energy needed to remove the highest energy electron from an isolated neutral atom in the gaseous state, for hydrogen it’s 1312 kJ/mol. • Ei decrease going down a group, goes up as move to right. Slight decrease at new p (main group 3), beginning new filled orbital (main group 6) Problem: ionization energies • Arrange the elements Se, Cl and S in order of increasing ionization energy. • Predict which element has the larger ionization energy. K or Br S or Te Higher ionization energies • Ionization is not limited to the loss of a single electron from an atom. Two, three or more electrons can be lost sequentially from an atom. • M + energy M+ + e- 1st ionization energy • M+ + energy M+2 + e- 2nd ionization energy • M+2 + energy M+3 +e- 3rd ionization energy • It takes larger amount of energy to remove more electrons than previous due to stronger pull of positively charge ion. Problem: higher ionization energies • What do you notice on the ionization chart as you get to a filled valence shell (s2p6)? • Which has the larger 5th ionization energy, Ge or As? • Which has the larger 3rd ionization energy, Be or N? Electron Affinity • The measure of energy change on adding an electron to an atom to form an anion. Ionization energies are always positive because energy is added to remove an electron (absorbed, endothermic). Electron affinities (Eea)generally negative because energy is released when a neutral atom adds an electron (released, exothermic). Electron Affinity chart (- kJ/mol) Trend in Eea • Halogens have large negative Eea for both high Zeff and room in shell. • Positive Eea are found for noble gas because the s and p are full. • Near zero Eea for alkaline earth metals due to filled s subshell. Problem: electron affinity • Why does nitrogen has a less favorable (more positive) Eea than its neighbors on either side, C and O? Ionic bonds and the formation of ionic solids • A tale of 2 elements: sodium and chlorine. Sodium and chlorine meet and sodium gives its electron to chlorine. Sodium forms a cation and chlorine forms an anion. They are attracted to one another by electrostatic forces and are joined by an ionic bond. The crystalline substance formed is an ionic solid, due to their communal living (sodium surrounded by other chlorines, and chlorine surrounded by other sodium) Energy changes between sodium and chlorine: Born-Haber cycle • Step 1: The conversion of solid Na metal into isolated, gaseous Na atoms, a process called sublimation. (+108 kJ/mol) • Step 2: The dissociation of gaseous Cl2 molecules into individual Cl atoms. Energy added to break appart. (+122 kJ/mol) • Step 3: The ionization of the isolated Na atoms into Na+ ions (+ 496 kJ/mol) • Step 4: The formation of Cl- ion (-349 kJ/mol) • Step 5: The formation of solid NaCl from isolated Na+ and Cl- (-788) Born-Haber diagram for NaCl Problem: Net energy calculations • Calculate the net energy change for the formation of KF(s) from the elements K(s) + ½ F2(g) KF (s) • Heat of sub K = 89.2 kJ/mol • Bond dissocition F2= 158 kJ/ mol • Electrostatic interaction in KF = -821 kJ/mol • Eea for F = -328 kJ/mol • Ei for K = 418.8 kJ/mol Lattice energies in ionic solids • The measure of electrostatic interaction energies between ion in a crystal and the measure of the strength of a crystal’s ionic bonds. • The force results is described by Coulomb’s law and is equal to the constant k times the charges on the ion, z1,z2 divided by the square of the distance between centers. • -U =F=k X (z1z2) / d2 Coulomb’s law • -U is the lattice energy Lattice energy Problem: lattice energies • Which has the larger lattice energy, NaCl or CsI? The octet rule • Main group elements tend to undergo reactions that leave them with eight outershell electrons, a noble gas electron configuration with filled s and p sublevels in their valence shells. • Exceptions occur toward the right side of the periodic table (groups 3-8) in the 3 period and below (NCl3 vs PCl5) Problem: chemical reactions and the octet rule • Lithium metal reacts with nitrogen to yield Li3N. What noble gas configuration does the nitrogen atom in Li3N have? End section 6a Chemistry of group 1A elements: Alkali metals Chemistry of group 2A elements: alkaline earth metals Chemistry of the Group 3A elements: Aluminum Chemistry of the Group 7A Elements: Halogens Chemistry of the group 8A elements: Noble gases