IS3
advertisement

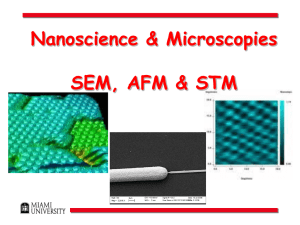
Slide 2: change reading to 6-11 Slide 5: added picture of Lavoisier, including Madam LaVoisier who performed many of the experiments and probably also played an important role in interpretation. Slide 7: added C70 allotrope. Comments, point out there are more! Slide 19: Organization by Electronic Structure Slide 20: reformated Slide 23: n+ l ordering rule given (to make sense of energy order) and referred to studio 3a to preframe. Slide 46: added reference. I could fix with embedded version if needed. Slide 48: inserted as summary of the SPM introduction Intersection 3 Reading: 1.4 p 6-11; 2.3-2.4 p 44-52; 2.9 p 62-68 [http://www.chemsoc.org/viselements/pages/history.html (history of the periodic table)] Outline • Elements and atoms • “The” periodic table • Seeing atoms today Elements __________ Atoms Subatomic particle Mass (g) Charge 9.10938188 x10-28 1.6762158 x10-24 1.67492716 x10-24 What makes an element an element? 0 Concept Question 1 Antoine Lavoisier, the "father of chemistry", listed lime as a chemical element in his table of 33 known elements. Which of the following observations best shows that lime cannot be an element? (a) Lime reacts with water, generating a large amount of heat. (b) Lime and carbon dioxide are produced when limestone is roasted. (c) When a certain soft metal is burned in oxygen, lime is produced (with no other products). (d) Lime melts at a temperature of 2572°C. Elements • Modern definition:An element is in this definition 'matter, all of whose atoms are alike in having the same positive charge on the nucleus.' Or, having the same number of protons in the nucleus. • The diatomic elements: – H, O, N, and the halogens (H2, O2, N2, F2, Cl2, Br2, I2) • Allotropes are different forms of the same element in the same physical state at the same temperature and pressure. – O2 vs. O3 – diamond, graphite, C60 buckyballs Four Allotropes of Carbon C60 “Buckyball” • • • C70 http://www.bris.ac.uk/Depts/Chemistry/MOTM/diamond/diamond.htm http://www.chem.yorku.ca/hall_of_fame/essays98/buckyball/bucky1/bucky.htm flex.ee.uec.ac.jp/ ~naka/fulleren.html IrReSPONSiBILiTiEs is the longest word that can be spelled entirely using chemical symbols without reusing any element... Ir - iridium Re - rhenium S - sulphur P - phosphorus O - oxygen N - nitrogen Si - silicon Bi - bismuth Li - lithium Ti - titanium Es – einsteinium How do you go from elements to the periodic table? Mendeleev's 1869 Periodic Table…. H1 Li 7 Ti 50 Zr 90 ? 100 V 51 Nb 94 Ta 182 Cr 52 Mo 96 W 186 Mn 55 Rh 104.4 Pt 197.4 Fe 56 Ru 104.4 Ir 198 Ni, Co 59 Pd 106.6 Os 199 Cu 63.4 Ag 108 Hg 200 Be 9.4 Mg 24 Zn 65.2 Cd 112 B 11 Al 27.4 ? 68 U 116 C 12 Si 28 ? 70 Sn 118 N 14 P 31 As 75 Sb 122 O 16 S 32 Se 79.4 Te 128? F 19 Cl 35.5 Br 80 I 127 Na 23 K 39 Rb 85.4 Cs 133 Tl 204 Ca 40 Sr 87.6 Ba 137 Pb 207 ? 45 Ce 92 Er? 56 La 94 Yt? 60 Di 95 Au 197? Bi 210? Mendeleev's 1871 Periodic Table…. R2O RO RH4 RO2 R2O3 RH3 R2O3 RH2 RO3 RH R2O7 RO4 H1 Li 7 Be 9.4 B 11 C 12 N 14 O 16 F 19 Na 23 Mg 24 Al 27.3 Si 28 P 31 S 32 Cl 35.5 K 39 Ca 40 ? 44 Ti 48 V 51 Cr 52 Mn 55 Cu 63 Zn 65 ? 68 ? 72 As 75 Se 78 Br 80 Rb 85 Sr 87 ? Yt 88 Zr 90 Nb 94 Mo 96 ? 100 Ag 108 Cd 112 In 113 Sn 118 Sb 122 Te 125 I 127 Cs 133 Ba 137 ? Di 138 ? Ce 140 ? ? ? ?,?,?,? ? ? ? ? ? ? ? ? ? ? ?Er 178 ??La 180 Ta 182 W 184 ? Au 199 Hg 200 Tl 204 Pb 207 Bi 208 ? ? ? ? ? Th 231 ? U 240 ? Fe, Co, Ni, Cu 56, 59, 59, 63 Ru, Rh, Pd, Ag 104, 104, 106, 108 Os, Ir, Pt, Au, 195,197,198,199 How can you organize the elements? • Observe the appearance, malleability, and reactivity of: – – – – – – – aluminum beryllium calcium lithium magnesium potassium sodium Two Columns (Groups) of Elements Describe any trends you observed in groups 1 and 2. • Rb and Cs are in the same column as Li, Na, and K on both the modern periodic table and Mendeleev's. What properties do you think that they have? Ask Ed if he will show you what happens when Rb and Cs are mixed with water. Writing chemical reactions The reaction between lithium and water forms a lithium hydroxide and hydrogen gas. The reaction gives off so much heat that it can ignite the hydrogen gas. Name Symbol State lithium water lithium hydroxide hydrogen ____Li(s) + ____H2O(l) ____LiOH(aq) + ____H2(g) Group 1 • Write balanced chemical equations for the reaction of sodium and potassium with water. • How do other metals such as copper and silver compare to the group 1 metals in physical properites and reactivity with water? Group 2 • What distinguishes the group 1 and group 2 metals? • Group 2 metals form hydroxides in a ratio of 1:2 that have chemical formula such as Mg(OH)2. Write balanced chemical equations for the reaction of the group two metals with water. Rows (Periods) • Describe how elements in rows are related. • Na, Mg, and Al are all in the same row. Predict the physical properties and reactivity of aluminum. • Are elements in groups or periods more alike? Organization by Electronic Structure Electron configurations H 1 e- 1s1 N 1 e- 1s22s22p3 He 2 e- 1s2 O 2 e- 1s22s22p4 Li 3 e- 1s22s1 F 3 e- 1s22s22p5 Be 4 e- 1s22s2 Ne 4 e- 1s22s22p6 B 5 e- 1s22s22p1 Na 5 e- 1s22s22p63s1 C 6 e- 1s22s22p2 Cl 6 e- 1s22s22p63s23p5 Concept Question 2 Which is the correct electron configuration for Zr? a) b) c) d) 1s22s22p63s23p64s23d104p65s24d2 1s22s22p63s43p64s23d104p65s24d2 1s22s22p63s23p64s23d104p65s25d2 1s22s22p63s23p64s23d104p64d4 Practice • Write the electron configurations for: – – – – P Sr Co As What do all of the Group 1 Elements have in common? • How would you describe “the” periodic table in relationship to electron configuration? Order = n + l with preference given to lowest value of n. Quantum numbers n and l discussed in studio 3a “The Atomic Hotel” Noble Gas Configuration • • Valence electrons: those in an atom's outer most shell (largest shell number) Electron configurations can get cumbersome (Ex. 107 electrons in Bohrium). Use the noble gas (group 8) core notation and write out only the valence electrons. – Examples: Mg 1s22s22p63s2 OR [Ne] 3s2 – Ru 1s22s22p63s23p64s23d104p65s24d6 OR [Kr]5s24d6 Practice • Write the electron configuration, using the noble gas configuration for: – – – – P Sr Co As Atomic Orbitals and the Periodic Table If you look at the valence electrons of each element in the same group, what do you find? Is there a single way to organize the elements? • In this song (Visual version) • What are some ways in which the elements were grouped? Other Periodic Tables How is this table organized? The Periodic Spiral of Professor Thoedor Benfey From The Pictorial Periodic Table by Chris Heilman Bayley, Thomsen, and Bohr: Seeing Atoms Today http://www.almaden.ibm.com/vis/stm/stm.html Electron Microscope 1930s Image ~12 atom molecule Must be used under vacuum Images from: www.vetref.net/ emscope/theorysch.html SEM Images Images from: www2.ijs.si/~goran/ semmate2.html STM (Scanning Tunneling Microscope) 1983 • STM 1986 Nobel Laureates Heinrich Rohrer and Gerd Binnig http://nobelprize.org/physics/educational/microscopes/scanning/ STM (Scanning Tunneling Microscope) 1. A tip is scanned over a surface at a distance of a few atomic diameters in a point-by-point and line-by-line fashion. At each point the tunneling current between the tip and the surface is measured. The tunneling current decreases exponentially with increasing distance and thus, through the use of a feedback loop, the vertical position of the tip can be adjusted to a constant distance from the surface. STM 2. The amount of these adjustments is recorded and defines a grid of values which can be displayed as a grayscale image. 3. Instead of assigning the values to a color we can also use them to deform the grid in the direction perpendicular to the surface. 4. Now we can bring back the grayscale and paint each square according to an average of the four defining grid points. STM images of elements and atoms Surface must be an electrical conductor nickel iron on copper copper http://www.almaden.ibm.com/vis/stm/atomo.html The Kanji characters for "atom." The literal translation is something like "original child." Macro models nano •1985 •Technique can directly resolve nanoscale features: 0.2nm in Z, 1-2nm in X, Y. • Can function in air and in fluids. How It Works • Laser beam is reflected off the back of the cantilever as it is raster scanned across the surface. • When tip encounters a feature on the surface, displacement of the cantilever results in displacement of the laser beam on the detector. • For example, if the tip scans across a feature projecting from the sample surface, the cantilever will deflect upwards and the resultant laser spot on the detector will be displaced lower. Heinz, William F.; Hoh, Jan H. J. Chem. Educ. 2005 82 695. Basic Mode 1: Contact Mode • In contact mode, the tip is constantly in touch with the sample surface. • Similar to a record player, the sample is moved underneath the tip. • The tip reads the “bumps and grooves” in the sample surface, only instead of converting these bumps and grooves into music, the AFM converts them into a topographic image. http://www.inkyfingers.com/RECORD/CECILEX/Cecil7.html Basic Mode 2: Tapping Mode Tapping mode, or intermittent contact mode, is often used for softer samples such as polymers or biological samples. Tip is oscillated by a piezoelectric crystal. As the tip approaches and interacts with the sample, the amplitude of the oscillation is decreased. A feedback loop measures the reduction in amplitude, which translates into a topographic image. Surface Characterization CD-R TappingModeTM AFM image of disc surface 10µm x 10 µm Image obtained from Digital Instruments website: http://www.di.com/NanoTheatre/theater.html Using AFM to Studio Programmed Cell Death (Apoptosis) -16 min. Height (µm) 16 min. Volume (um3) 3500 3000 48 min. 1 hr 20 min. 1 hr 52 min. 2 hrs 24 min. 2500 2000 Distance (µm) 1500 0 20 40 60 80 100 120 140 Time (min.) J. Hessler et al. Langmuir 2005, 21, 9280-9286. More AFM Images Zhao,Y.; Chen, Z.; Yuan,H.; Gao, X.; Qu, L.; Chai, Z.; Xing, G.; Yoshimoto, S.; Tsutsumi, F.; Itaya, K. “Highly Selective and Simple Synthesis of C2m-X-C2n Fullerene Dimers” J.Am. Chem. Soc. 2004, 126, 11134 – 11135. Seeing Atoms Today Scanning Probe Microscopes (STM and AFM) allow us to explore chemistry at an atom by atom level as well as in the 11000 nm size scale. What happened to the man who was stopped for having sodium chloride and a nine-volt in his car? He was booked for a salt and battery. Concept Question 3 What is the approximate number of carbon atoms it would take placed next to each other to make a line that would cross this dot: • a. 4 b.200 c. 30,000,000 d.6.02 x 1023