Protease inhibitors in chronic hepatitis C - Liver
advertisement

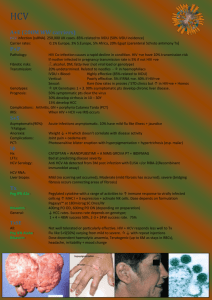
Protease Inhibitors in Chronic Hepatitis C: An Update Chapter 1 – Management of Hepatitis C: Updated Guidelines from the Canadian Association for the Study of the Liver (CASL) Edited by Morris Sherman MD BCh PhD FRCP(C) Associate Professor of Medicine University of Toronto November 2012 Management of Hepatitis C: Updated Guidelines from the Canadian Association for the Study of the Liver (CASL) Robert P. Myers, MD, MSc Associate Professor, Liver Unit Division of Gastroenterology University of Calgary Objectives: HCV Management Review updated CASL recommendations for management of HCV genotype 1* Burden of HCV in Canada Pre-treatment assessment Triple therapy including boceprevir and telaprevir Adverse effects Drug-drug interactions Antiviral resistance * Recommendations for non-1 genotypes are unchanged from the 2007 CASL HCV guidelines. Burden of HCV in Canada Significant medical and economic burden Seroprevalence unknown Risk Group Population Prevalence Prevalent Cases Proportion of Cases 268,200 52% 140,000 58% Current IDU 84,400 62% 52,500 22% Previous IDU 183,800 48% 87,500 36% Transfusion 3,325,700 0.8% 25,900 11% Hemophilia 2,200 40% 900 0.4% Other 27,624,300 0.27% 75,800 31% Total 31,220,500 0.8% 243,000 100% IDU, total Remis RS. PHAC 2007 Burden of HCV in Canada Modelled incidence ~8,000 incident cases annually (80% IDUs) Proportion diagnosed unclear (<80%) HCV-related complications rising Insufficient manpower to treat all cases 900 Cirrhosis 800 700 600 Decomp 500 400 HCC 300 Transplant 200 100 0 1967 1972 1977 1982 1987 1992 1997 2002 2007 2012 2017 2022 2027 Year Remis et al. PHAC 2007 Liver-related death vs. no treatment (%) Antiviral Therapy Must be Maximized to Make an Impact 100 90 80% SVR rate 60% SVR rate 40% SVR rate 34% ↓ 80 68% ↓ 70 60 50 40 30 20 0 Current* 25% 50% 75% 100% Proportion of population treated * Assumes 30% Dx & up to 25% Rx’d in 2010. Outcomes at 2020. Davis GL et al. Gastroenterology 2010; 138(2):513-21 Burden of HCV in Canada: CASL Recommendations A large population-based seroprevalence survey should be conducted to accurately define the prevalence of hepatitis C in Canada. The design of the study should include populations with an increased risk of hepatitis C, particularly IDUs and immigrants from endemic countries. Increased resources are necessary to improve hepatitis C treatment capacity in Canada, including the training of expert treaters and public funding for treatment nurses. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Who Should Be Treated? CASL Recommendations Myers RP et al. Can J Gastro 2012; 26(6):359-75 Who Should Be Treated? CASL Recommendations All patients with chronic HCV, particularly those with liver fibrosis, should be considered candidates for antiviral therapy. Patients with extrahepatic manifestations of HCV should be considered for antiviral therapy. Persistently normal ALT does not exclude significant liver disease nor preclude the need for antiviral therapy. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Pre-Treatment Assessment: Is Liver Biopsy Really Necessary? Some fibrosis assessment necessary Prognosis Necessity of treatment Surveillance for HCC & varices F2 threshold less important with improved therapies Biopsy is imperfect Sampling error; variability in pathologic interpretation Numerous noninvasive alternatives to biopsy Bedossa P et al. Hepatology 2003; 38(6):1449-57 Pre-Treatment Assessment: Non-invasive Measures of Fibrosis Test (Reference) Components Cut-off F2-F4 vs. F0-F1 Sensitivity/specificity F2-F4 vs. F0-F1 FibroScan (Castera, 2005) Liver stiffness by transient elastography ≥7.1 kPa 67% / 89% APRI (Shaheen, 2007) AST/ULN x 100 Platelets ≥0.5 ≥0.7 ≥1.5 81% / 50% 84% / 70% 35% / 91% FibroTest (Poynard, 2004) α2M, haptoglobin, apo-A1, GGT, bilirubin ≥0.58 56% / 83% α2M, HA, TIMP-1 ≥0.36 77% / 73% α2M, HA, GGT, bilirubin ≥0.50 89% / 63% α2M, HA, AST, platelets, PT, urea ≥0.50 75% / 78% FibroSpect II (Patel, 2004) Hepascore (Adams, 2005) FibroMeter (Leroy, 2005) Pre-Treatment Assessment: CASL Recommendations Assessment of Disease Severity All patients with HCV should have an assessment for the severity of liver fibrosis. Acceptable methods include liver biopsy, TE (FibroScan), and serum biomarker panels (e.g. APRI, FibroTest, Fibrometer), either alone or in combination. Alternatively, cirrhosis can be confidently diagnosed in some patients with clear clinical or radiographic evidence. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Pre-Treatment Assessment: CASL Recommendations Virologic Testing HCV RNA and genotype testing are essential to the management of patients with chronic hepatitis C. HCV RNA testing should be performed using a sensitive quantitative assay (lower limit of detection ≤ 10-15 IU/mL) with a broad dynamic range. Standardized results should be expressed in IU/mL and be available within a maximum of 7 days in order to facilitate management decisions. Although genotype 1b has higher response rates vs. genotype 1a, testing for HCV subtype is not indicated This may change with newer DAAs available in the future Myers RP et al. Can J Gastro 2012; 26(6):359-75 Interleukin 28B (IL28B) Associated with viral clearance ~50% of ethnic variation in SVR rates Strongest pre-treatment predictor of SVR, but on-treatment response more important 100 P=1.06x10-25 80 SVR (%) Single-nucleotide polymorphisms (SNPs) on chromosome 19 Encodes IFN-λ3 P=2.06x10-3 P=4.39x10-3 P=1.37x10-28 Numbers on bars represent n 60 40 20 102 433 336 0 70 91 30 14 35 26 186 559 392 T/T T/C C/C T/C C/C T/C C/C T/C C/C EuropeanAmericans AfricanAmericans Hispanics Combined rs12979860 SVR (%) Non-SVR (%) Ge. Nature 2009. Suppiah. Nat Genet 2009. Tanaka. Nat Genet 2009. Thomas. Nature 2009. Pre-Treatment Assessment: CASL Recommendations IL28B Genotyping The IL28B genotype may provide valuable information regarding the likelihood of SVR and the probability of qualifying for shortened treatment duration in previously untreated patients with genotype 1. The role of IL28B genotyping is limited in treatmentexperienced patients and those with genotypes other than 1 and 4. A non-favourable IL28B genotype does not preclude antiviral therapy. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Antiviral Therapy for HCV Genotype 1: CASL Recommendations Triple therapy including peginterferon (PEG-IFN), ribavirin (RBV), and a protease inhibitor (telaprevir or boceprevir) is the new standard of care in treatment-naïve and previous treatment failures. Boceprevir (800 mg every 8 hours with food) is administered after a 4-week lead-in period of PEG-IFN and RBV. Duration of therapy depends on patient characteristics and treatment response. Telaprevir (750 mg every 8 hours with non-low fat food) should be started simultaneously with PEG-IFN and RBV and given for the initial 12 weeks of therapy. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Response-Guided Therapy (RGT): CASL Recommendations RGT - the tailoring of treatment duration based on early viral kinetics - can be employed in selected patient subgroups. Boceprevir: HCV RNA negative at weeks 8 through 24 Telaprevir: HCV RNA negative at weeks 4 through 12 SVR rates of ~90% have been reported with 24 to 28 weeks of therapy in patients qualifying for RGT. Partial responders treated with telaprevir, patients with cirrhosis, and prior null responders should not receive RGT. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Adherence to Antiviral Therapy: CASL Recommendations Adherence to treatment and to futility rules, and close monitoring of concomitant drugs and side effects are particularly important with PI-based therapy. Optimal management of this population should be conducted by well-trained, experienced personnel. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Futility Rules: CASL Recommendations Strict adherence to futility rules is vital to limit exposure to potential side effects of these costly therapies that will not achieve SVR, and to reduce emergence of antiviral resistance. All therapy – including PEG-IFN and RBV – must be discontinued if futility rules are met: Boceprevir: HCV RNA ≥100 IU/mL at week 12 or detectable at week 24 Telaprevir: HCV RNA >1,000 IU/mL at week 4 or 12, or detectable at week 24 Identical futility rules apply to treatment-naïve and treatment-experienced patients. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Futility Rules Indicate Treatment Failure Even if the Viral Load Has Declined 107 1,800,000 HCV RNA (IU/mL) 106 99.9% reduction: Continue? 105 104 1,230 103 475 102 If futility rules met, RNA is rising! Stop therapy! 10 1 W0 W1 W2 W3 W4 Slide courtesy of Dr. J. Feld. Adverse Effects of the Protease Inhibitors (PIs) PI-based therapy associated with more adverse effects than PEG-IFN and RBV dual therapy No data to support switching from one PI to another to manage toxicity Major adverse effects differ by PI Boceprevir: anemia (~50%), dysgeusia (~40%) Telaprevir: anemia (~40%), rash (~40%), anorectal symptoms (~30%) Myers RP et al. Can J Gastro 2012; 26(6):359-75 Adverse Effects of the Protease Inhibitors (PIs): CASL Recommendations Treatment with PIs should be supervised by experienced personnel and adverse effects monitored closely. Close monitoring of hemoglobin levels is essential during antiviral treatment for HCV, particularly during the administration of PIs. Management of anemia may include any of the following strategies: RBV dose reduction (first line), transfusion of packed red blood cells, and/or erythropoietin administration. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Drug-Drug Interactions (DDIs) Boceprevir and telaprevir are substrates and inhibitors of CYP3A4* CYP3A4 metabolizes many common drugs Potential increased drug concentrations with PI coadministration Drugs that induced CYP3A4 may reduce PI concentration (i.e. antiviral treatment efficacy) Numerous potential DDIs with PI-based therapy Antiarrhythmics, anticoagulants, anticonvulsants, antihistamines, antibacterials, antiretrovirals, statins, herbal products, immunosuppressants, OCPs, phosphodiesterase inhibitors, and some sedatives/hypnotics * Minor elimination pathways include P-glycoprotein and aldoketoreductase. Drug-Drug Interactions (DDIs): CASL Recommendations Prior to the initiation of PIs, potential DDIs must be considered, including those attributable to prescription and over-the-counter pharmaceuticals and herbal preparations. Review product monographs and useful online resources for potential DDIs prior to initiating therapy. http://www.hep-druginteractions.org/ http://medicine.iupui.edu/clinpharm/ddis/ Myers RP et al. Can J Gastro 2012; 26(6):359-75 Antiviral Resistance HCV RNA change from baseline (Log10 IU/mL) All resistance variants pre-exist Not caused by PIs, but unmasked by selective pressure Reflect inadequate response to PEG-IFN/RBV Predominant cause (80-90%) of incomplete viral suppression, breakthrough, or relapse Genotype 1a > 1b 1 Modest or null IFNa-ribavirin effect 0 -1 -2 Resistant HCV -3 -4 Wild-type, sensitive HCV -5 Study time Pawlotsky JM. Hepatology. 2011 May; 53(5):1742-51 Antiviral Resistance: CASL Recommendations In order to reduce the development of antiviral resistance to the PIs, patients who meet futility rules indicating a high likelihood of treatment failure should discontinue therapy immediately. Dosage reductions of boceprevir and telaprevir should not be utilized to manage treatment-related side effects. To prevent resistance, PIs must be stopped if either PEG-IFN or RBV are discontinued. There is no role for pre-treatment resistance testing. Myers RP et al. Can J Gastro 2012; 26(6):359-75 Summary: CASL Guidelines for the Management of HCV Must maximize case-finding, referral, and antiviral Rx to reduce HCV burden in Canada. Barriers to treatment (e.g. need for biopsy) should be minimized. New therapies (boceprevir and telaprevir) markedly improve SVR rates in genotype 1 (treatment-naïve and experienced), but are complex and have additional side effects. The Canadian Liver Foundation gratefully acknowledges the participating health care professionals for their contributions to this project and for their commitment to the liver health of Canadians. The Canadian Liver Foundation (CLF) was the first organization in the world devoted to providing support for research and education into the causes, diagnoses, prevention and treatment of all liver disease. Through its chapters across the country, the CLF strives to promote liver health, improve public awareness and understanding of liver disease, raise funds for research and provide support to individuals affected by liver disease. For more information visit www.liver.ca or call 1-800-563-5483. This project made possible through the financial support of Merck Canada Inc. The views, information and opinions contained herein are those of the authors and do not necessarily reflect the views and opinions of Merck Canada Inc.