Adiabatic Runaway Scenario
advertisement

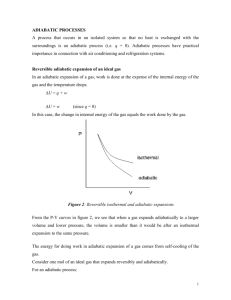
Estimation of Time to Maximum Rate under Adiabatic Conditions (TMRad) Using Kinetic Parameters Derived from DSC - Investigation of Thermal Behavior of 3-methyl-4-nitrophenol APSS 2009 20-23 October, 2009, Osaka, Japan Bertrand Roduit1, Franz Brogli2, Francesco Mascarello3, Mischa Schwaninger3, Thomas Glarner4, Jacques Wiss5, Markus Luginbühl6, Craig Williams6, Pierre Reuse7 1AKTS AG Advanced Kinetics and Technology Solutions, TECHNOArk 1, 3960 Siders, Switzerland 2Ciba Schweizerhalle AG, P.O. Box, CH-4002 Basel, Switzerland 3DSM Nutritional Products Ltd., Safety laboratory, 4334 Sisseln, Switzerland 4F. Hoffmann-La Roche Ltd, Safety laboratories, 4070 Basel, Switzerland 5Novartis Pharma AG, Novartis Campus, WSJ-145.8.54, 4002 Basel, Switzerland 6Syngenta Crop Protection Münchwilen AG, WMU 3120.1.54, 4333 Münchwilen, Switzerland 7Swiss Safety Institute, Schwarzwaldallee 215, WRO-1055.5.02, 4002 Basel, Switzerland www.akts.com 1 Adiabatic Runaway Scenario Example of adiabatic runaway scenario Before : 2 Adiabatic Runaway Scenario Example of adiabatic runaway scenario After : 3 Analysis Samples Analysis samples: 3-methyl-4-nitrophenol CAS No: 2581-34-2 Objective: Determine the initial temperature for Time To Maximum Rate under adiabatic conditions TMRad = 24h - Different suppliers (different batches) - DSC or ARC techniques were applied - Different DSC apparatus (various manufacturers) 4 3-methyl-4-nitrophenol at 4 K/min 5 Exo Heat : -2,194.016 (J/g) T : 198.92 and 344.13 (°C) Top of Peak : 294.34 (°C) Peak Height : 4.41 (W/g) Baseline Type : Tangential Sigmoid 4 HeatFlow (W/g) 3 2 1 0 -1 -2 -3 128.16 (°C) -4 125 . 150 175 200 225 250 Temperature (°C) 275 300 325 Typical DSC trace of 3-methyl-4-nitrophenol recorded at 4 K/min and sigmoid baseline construction. 5 Reproducibility of the DSC traces Reaction rate (-/s) 2E-3 1.5E-3 1E-3 5E-4 0 200 . 220 240 260 280 Temperature (°C) 300 320 340 The reaction rates for all samples at 4K/min. Despite of the different experimental setups and sample origins the reproducibility of the DSC traces is acceptable. 6 Differential isoconversional method Theory: isoconversional analysis & baseline optimization 2 2 1 3 3 = E 1 d ln Const R T dt ln 7 Reactions rate and progress: Non-isothermal Reaction rate (-/s) Reaction rates d/dt and progresses corresponding to the normalized DSC-signals for the decomposition of all 3-methyl-4-nitrophenol samples under non-isothermal conditions. The values of the heating rates are marked on the curves. The comparison of the experimental and simulated signals at chosen experimental conditions is shown in the respective insets. 0.003 4 8 0.002 4 . 1 0.001 2 0.5 0.25 0 1 Reaction progress (-) 0.25 0.5 0.8 1 2 0.6 4 8 0.4 4 4 0.2 . 0 200 . 220 240 260 280 Temperature (°C) 300 320 340 8 Reactions rate and progress: Isothermal Reaction rates d/dt and progresses corresponding to the normalized DSC-signals for the decomposition of all 3-methyl-4-nitrophenol samples under isothermal conditions. The values of the temperatures are marked on the curves. The comparison of the experimental and simulated signals at chosen experimental conditions is shown in the respective insets. 260 6E-4 220 Reaction rate (-/s) 5E-4 250 4E-4 240 3E-4 2E-4 . 230 220 1E-4 210 200 190 0 Reaction progress (-) 1 260 250 0.8 240 230 0.6 220 220 210 0.4 200 0.2 . 190 0 0 . 2 4 6 8 Time (h) 10 12 14 16 9 Experimental Validation Isothermal validation ARC validation Initial temperature for TMRad 24h = ? °C 10 Link between kinetics and TMRad Determination of time to maximum rate under adiabatic conditions (TMRad) Adiabatic Conditions Or = From DSC > 1000 kg Theory 11 Key parameters in adiabatic experiments Theory Temperature /°C Temperature profile of an adiabatic runaway reaction, DTadiabatic Determination of time to maximum rate under adiabatic conditions (TMRad) Key parameters obtained from adiabatic experiments Time /h 12 Key parameters in adiabatic experiments Theory Temperature /°C Time to Maximum Rateadiabatic Maximum Selfheat rate Selfheat rate /°C/min Temperature profile of an adiabatic runaway reaction, corresponding self-heating rate DTadiabatic Determination of time to maximum rate under adiabatic conditions (TMRad) Key parameters obtained from adiabatic experiments Time /h 13 Experimental Validation Typical ARC test for 3-methyl-4-nitrophenol carried out in HWS mode. Having the kinetic description of the reaction rate from the DSC data, one can estimate that the reaction progress a after ca. 11.3 h of HWS testing amounts to about 0.0095 (ca. 1%). From the time at which the temperature of the detection limit (183.81°C) was reached the value of TMR amounts to ca. 4.4h (15.67-11.29h). Solid line depicts the simulation being in a good agreement with the experimental HWS-ARC data presented as symbols. 500 450 t: 15.67 (h) T: 421.65 (°C( 400 300 250 200 150 t: 11.29 (h) T: 183.81 (°C) 100 50 1 0.8 0.6 0.4 0.2 0 Reaction Progress: 0.0095 (-) 0 2 4 6 8 Time (h) 10 12 14 Reaction Progress (-) Temperature (°C) 350 16 14 Experimental Validation Isothermal validation ARC validation Initial temperature for TMRad 24h = ? °C (F =1) 15 TMRad 24 h Summary of the results of determination of the initial temperatures leading to TMRad = 24 h with AKTS-Thermokinetics Software by using all DSC data collected in round robin test. Participant of round robin test Heating rates applied (non-isothermal) Temperatures applied (isothermal) 0.25, 0.5, 1, 2, 4 200, 210, 220, 240 0.5, 1, 2, 4, 8 DHr ± s Initial temperature for TMRad = 24 h Sum of all correl. coeff. 1961.2± 151.8 156.4 9960 2070.5 ± 166.7 153.6 9894 4, 4 220, 240, 260 2143.2 ± 115.1 148.9 9932 0.5, 2, 4, 8 210, 220, 230, 240 2133.8 ± 144.7 149.4 9934 148 2.5, 2.5 190, 200, 210, 220 5 220, 230, 240, 250, 260 2112.1 ± 76.5 152.5 9978 1655.8 ± 141.9 150.1 9972 Mean value for TMRad 24h = 151.27 ±3.01°C 16 Experimental Validation Isothermal validation ARC validation Initial temperature for TMRad 24h = 151°C (F =1) 17 Conclusion ‘Safety through calculations not by accidents’ The correct determination of TMRad based on DSC data requires two important parameters (i) an advanced kinetics of the investigated reaction and (ii) an adiabatic heat balance of the system. 18 Advanced Kinetics and Technology Solutions Acknowledgements Our partners and friends AKTS AG, C. Borgeat, C. Luyet, L.Xia, N. Solioz, JG. Pont armasuisse, Dr. P. Folly, Dr. A.Sarbach and B. Berger Swiss Federal office of Public Health, Dr. V. Dudler Univ. of Western Switzerland, Prof. J.N. Aebischer, S. Gomez, B. Alonso Swiss Institute of Safety and Security, Dr. P. Reuse, Prof. F. Stoessel, Dr. H. Fierz Nitrochemie Wimmis AG, Dr. M. Ramin, Dr. U. Schädeli, Dr. B. Vogelsanger 19