Document
advertisement

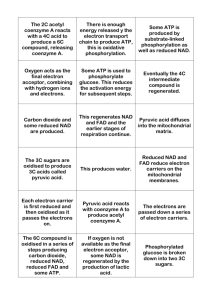
Coenzymes • Many enzymes contain small nonprotein molecules and metal ions • Coenzyme – a complex organic or metalloorganic molecule • Prosthetic groups – are distinguished by their tight, stable incorporation into a protein’s structure by covalent or noncovalent forces. – Examples • Pyridoxal phosphate,flavin mononucleotide (FMN), flavin dinucleotide (FAD), thiamin pyrophosphate, biotin, and the metal ions. • Some enzymes require both a coenzyme and one or more metal ions for activity • Coenzymes – participate directly in substrate binding or catalysis. • A complete, catalytically active enzyme together with its bound coenzyme and/or metal ions is called a holoenzyme. The protein part of such an enzyme is called the apoenzyme or apoprotein. • Intracellular range of concentration – 0.01 -1.0 meq L-1 • Coenzymes may be covalently bound to the enzyme or free to associate and dissociate from the protein. • may facilitate the binding and orientation of substrates • the formation of covalent bonds with reaction intermediates (Co2+ in coenzyme B12) • interaction with substrates to render them more electrophilic (electron-poor) or nucleophilic (electron-rich). • Coenzymes serve as recyclable shuttles—or group transfer reagents—that transport many substrates from their point of generation to their point of utilization. – Other chemical moieties transported by coenzymes include methyl groups (folates), acyl groups (coenzyme A), and oligosaccharides (dolichol). • Association with the coenzyme also stabilizes substrates such as hydrogen atoms or hydride ions that are unstable in the aqueous environment of the cell. • Chemical reactions of many types take place between substrates and enzymes’ functional groups (specific amino acid side chains, metal ions, and coenzymes). • Catalytic functional groups on an enzyme may form a transient covalent bond with a substrate and activate it for reaction, or a group may be transiently transferred from the substrate to the enzyme. • A number of amino acid side chains, and the functional groups of some enzyme cofactors can serve as nucleophiles in the formation of covalent bonds with substrates. • The covalent bond formed can activate a substrate for further reaction Coenzyme role • Epimerization involves an oxidation and reduction at carbon 4 with NAD+ as coenzyme. • Each step in fatty acid oxidation involves acylCoA derivatives catalyzed by separate enzymes, utilizes NAD+ and FAD as coenzymes, and generates ATP • Susceptibility to proteolytic degradation can be influenced by the presence of ligands such as substrates, coenzymes, or metal ions that alter protein conformation • NAD is also required for the poly-ADP-ribose polymerase reaction, which is part of the cellular DNA damage recognition system and regulates DNA replication, DNA repair, and cell cycle progression. • Metabolic pathways are regulated at several levels, from within the cell and from outside – The most immediate regulation is by the availability of substrate – A second type of rapid control from within is allosteric regulation by a metabolic intermediate or coenzyme—an amino acid or ATP, for example—that signals the cell’s internal metabolic state. • Many coenzymes, cofactors, & prosthetic groups are derivatives of B vitamins • Many coenzymes contain, in addition, the adenine, ribose, and phosphoryl moieties of AMP or ADP. Many coenzymes and related compounds are derivatives of adenosine monophosphate. Synthesis • They are synthesized by a variery of ammalian cell types • Synthesis of nucleotide coenzymes is regulated so that there are essentially constant concentrations of these coenzymes in the cell. • Synthesis of niacin requires pyridoxine, riboflavin, and iron. • The function of coenzymes is chemically varied, and we describe each separately • The coenzyme pyridoxal phosphate (PLP) is present at the catalytic site of aminotransferases and of many other enzymes that act on amino acids • In each case, the thiamin diphosphate provides a reactive carbon on the thiazole moiety that forms a carbanion, which then adds to the carbonyl group of, for instance, pyruvate. The addition compound then decarboxylates, eliminating CO2. • Electrical stimulation of nerve leads to a fall in membrane thiamin triphosphate and release of free thiamin. It is likely that thiamin triphosphate acts as a phosphate donor for phosphorylation of the nerve membrane sodium transport channel. • NAD Is the Source of ADP-Ribose – ADP-ribosylation – DNA repair mechanism • Vitamin B6 Has Several Roles in Metabolism – transamination and decarboxylation – glycogen phosphorylase, where the phosphate group is catalytically important – in steroid hormone action • where it removes the hormone-receptor complex from DNA binding, terminating the action of the hormones. • A few types of coenzymes and proteins serve as universal electron carriers • Both NAD and NADP accept two electrons and one proton. • the coenzymes function catalytically and are recycled repeatedly without a net change in the concentration of NAD NADH. • NAD and NADP are the freely diffusible coenzymes of many dehydrogenases. • NAD or NADP accepts a hydride ion (:H-, the equivalent of a proton and two electrons) • the coenzymes function catalytically and are recycled repeatedly without a net change in the concentration of NAD+ + NADH. • NAD and NADP are bound to dehydrogenases in a widely conserved structural motif called the Rossmann fold. • Coenzymes play key roles In the citric acid cycle – energy-yielding metabolism • NAD(P)+-dependent dehydrogenases are assayed spectrophotometrically – the rate of change in optical density at 340 nm will be proportionate to the quantity of enzyme present • Many enzymes that do not directly reduce NAD+ or FAD generate products that can be acted upon by a NAD(P) or FAD-linked dehydrogenase. Thus by coupling two enzyme reactions Sirtuins • The sirtuins are a highly conserved family of NAD+-dependent enzymes • sirtuins serve as the bridge between what we eat and what we are. • Sirtuin activity is intimately tied to the metabolic state of the cell. – Linking Chromatin Remodeling to Metabolism • requires NAD+ cleavage with each reaction cycle • flavoproteins as electron carriers • Certain flavoproteins act in a quite different role as light receptors. – Mediate the effects of light on mammalian circadian rhythms (oscillations in physiology and biochemistry, with a 24-hour period) • Photolyases use the energy of absorbed light to repair chemical defects in DNA. • Vitamin C Is the Coenzyme for Two Groups of Hydroxylases – Copper-containing hydroxylases – α-ketoglutarate-linked iron-containing hydroxylases CLINICAL CORRELATION • Cystathioninuria • Mutation of a coenzyme-binding site results in clinical disease – y-cystathionase • Cystathionine → cysteine + α-ketobutyrate • the Km for pyridoxal phosphate binding to the enzyme was increased Arsenic Poisoning • For the most part, arsenic poisoning is explained by inhibition of enzymes that require lipoic acid as a coenzyme. These include pyruvate dehydrogenase, αketoglutarate dehydrogenase, and branchedchain a-keto acid dehydrogenase. Deficiency of vitamines • limited diets, when food is cooked at high temperatures for long periods, • intestinal diseases, Inability to absorb • in newborns , pregnant women • Folic Acid Deficiency – Altered appearance of blood cells and formiminoglutamate excretion • methyl malonic aciduria – Acidosis – 5’-adenosylcobalamin deficiency (coenzyme of methyl malonyl CoA isomerization). • methylmalonic aciduria Assay of vitamines(coenzyme) • by measuring one or more enzyme activities in isolated red blood cells As a tool for enzyme Purification • Stationary phase matrices available commercially contain ligands such as NAD+ or ATP analogs. • Bound proteins are then eluted either by competition with soluble ligand or, less selectively, by disrupting protein-ligand interactions using urea, guanidine hydrochloride, mildly acidic pH, or high salt concentrations.