Chemical Nomenclature: Naming Compounds & British vs. American Terms
advertisement

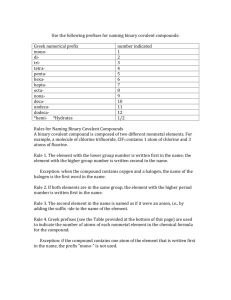
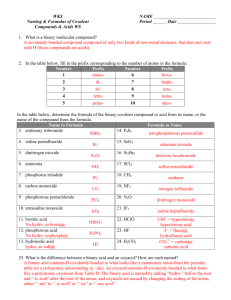
What’s in a name? • When two people use different names for the same thing, misunderstood words are apt to happen. The British and Americans often get confused. American English American English Find the bonnet. British English British English Find the boot. Which is the chemist and which is the pharmacist? In Ireland and England, the people who Americans call pharmacists are called chemists British English American English Plaster: American English Plaster: British English Which is plaster and which is a band-aid? Kerosene: American English Paraffin: British English Paraffin: American English Nomenclature • A System of Naming Compounds • Compounds are two or more atoms of different elements bonded together. • Even though the gases O2, N2, F2, and Cl2 travel in pairs, their names are simply oxygen, nitrogen, fluorine, and chlorine. METALS vs. Non-METALS F2 & Cl2 (Fluorine & chlorine gas) HF(g) Hydrogen Fluoride HCl(g) Hydrogen Chloride H2S Dihydrogen Sulfide HCN – Hydrogen cyanide NO - NO2- NO3 - N2O Cl2O dichlorine monoxide NH3 Ammonia PCl3 Phosphorus trichloride In order to be effective in using prefixes to name compounds containing two non-metals, these prefixes must be committed to memory: mono- 1 di2 tri3 tetra- 4 penta- 5 hexa- 6 hepta- 7 octa8 nona9 deca- 10 undeca- 11 dodeca- 12 Example #1-Names to Formulas I’m a Binary Compound Sulfur trioxide 1. Write symbols of elements 2. Write number of atoms S1O3 If no prefix, then 1 is implied andFormula not written Final Example #2-Names to Formulas I’m a Binary Compound dichlorine heptaoxide 1. Write symbols of elements 2. Write number of atoms Cl2O7 Final Formula Example #3-Names to Formulas I’m a Binary Compound oxygen difluoride 1. Write symbols of elements 2. Write number of atoms O1F2 If no prefix, then 1 is Formula implied Final and not written Example #4-Names to Formulas I’m a Binary Compound dinitrogen tetraoxide 1. Write symbols of elements 2. Write number of atoms N2O4 Final Formula Example #5-Names to Formulas I’m a Binary Compound phosphorus pentachloride 1. Write symbols of elements 2. Write number of atoms P1Cl5 If no prefix, then 1 is Final implied andFormula not written Example #6-Names to Formulas I’m a Binary Compound dinitrogen monoxide 1. Write symbols of elements 2. Write number of atoms N2O 1 If no prefix, then 1 is Final implied andFormula not written Example #7-Names to Formulas I’m a Binary Compound carbon monoxide 1. Write symbols of elements 2. Write number of atoms C1O 1 If no prefix, then 1 is Final implied andFormula not written Example #8-Names to Formulas I’m a Binary Compound sulfur hexafluoride 1. Write symbols of elements 2. Write number of atoms S1F6 If no prefix, then 1 is Final implied andFormula not written Example #10-Names to Formulas I’m a Binary Compound dinitrogen trisulfide 1. Write symbols of elements 2. Write number of atoms N 2S 3 Final Formula Examples #1- Formulas to Names 1. Write names of elements 2. Write number of atoms CCl4 I’m a Binary Compound monocarbon tetra chloride ine If first prefix is mono, it is Final Name implied and not written Examples #2- Formulas to Names 1. Write names of elements 2. Write number of atoms XeF3 monoxenon I’m a Binary Compound ide tri fluor ine If first prefix is mono, it is Final Name implied and not written Examples #4- Formulas to Names 1. Write names of elements 2. Write number of atoms AsI3 I’m a Binary Compound ine monoarsenic triiod ide If first prefix is mono, it is Final Name implied and not written Examples #6- Formulas to Names 1. Write names of elements 2. Write number of atoms IF5 I’m a Binary Compound ine monoiodine pentafluoride If first prefix is mono, it is Final Name implied and not written Examples #8- Formulas to Names 1. Write names of elements 2. Write number of atoms N2S5 I’m a Binary Compound di nitrogen pentasulfide ur Final Name Examples #9- Formulas to Names 1. Write names of elements 2. Write number of atoms SiCl4 I’m a Binary Compound ine monosilicon tetrachloride If first prefix is mono, it is Final Name implied and not written Practice Problem #1 ClF3 Choose the correct name for the compound 1. carbon iodine trifluoride 2. chlorine trifluorine 3. chlorine trifluoride 4. chlorine tetrafluoride 5. none of the above Element List Prefixes next problem Practice Problem #2 arsenic pentabromide Choose the correct formula for the compound 1. AsBr5 2. ArBr5 3. AsBr7 4. As5Br 5. none of the above Element List Prefixes next problem Practice Problem #3 N2O3 Choose the correct name for the compound 1. nitrogen trioxide 2. dinitride trioxide 3. dinitrogen trioxygen 4. dinitrogen trioxide 5. none of the above Prefixes next problem Practice Problem #6 dichlorine monoxide Choose the correct formula for the compound 1. ClO 2. Cl2O 3. ClO2 4. Cl2O2 5. none of the above Prefixes next problem Practice Problem #8 Tetraphosphorus decaoxide Choose the correct formula for the compound 1. P4O10 2. P6O10 3. 4PO10 4. P2O5 5. none of the above Prefixes next problem Practice Problem #11 NO Choose the correct name for the compound 1. mononitrogen monoxide 2. nitrogen monoxygen 3. mononitrogen oxide 4. nitride monoxide 5. none of the above This is nitrogen monoxide next problem Practice Problem #16 silicon tetrabromide Choose the correct formula for the compound 1. SBr4 2. ScBr4 3. SiB4 4. SiBr6 5. none of the above Element List Prefixes The correct formula is SiBr4 next problem Practice Problem #17 S4N4 Choose the correct name for the compound 1 tetrasulfur tetranitride 2. tetrasulfur nitride 3. trisulfur trinitride 4. tetrasulfur tetranitrogen 5. none of the above Prefixes next problem Practice Problem #19 BF3 Choose the correct name for the compound 1. monoboron trifluoride 2. boron trifluorine 3. boron trifluoride 4. bromine trifluoride 5. none of the above Element List Practice Problem #20 tetraphosphorus decasulfide Choose the correct formula for the compound 1. P6S9 2. P4S9 3. P4Si10 4. Ph4S10 5. none of the above Element List is Prefixes The correct formula P4S10 next problem dual properties H H B Al Si Ge As Sb Te Po At Element List 1 Actinium Ac 24 Fluorine F 47 Lanthanum La 70 Plutonium Pu 2 Aluminum Al • Fr 48 Lead Pb 71 Polonium Po 4 Argon Ar 24 Gallium Ga 49 Lithium Li 72 Potassium K 5 Arsenic As • Germanium Ge 50 Magnesium Mg 73 Radium Ra 6 Astatine At • Gold Au 51 Manganese Mn 74 Radon Rn 7 Barium Ba • Helium He 52 Mercury 9 Beryllium Be • Hygrogen H 53 Molybddenum Mo 10 Bismuth Bi • Indium In 54 Neon Ne 12 Boron B • Iodine I 55 Nickel Ni 13 Bromine Br • Iridium Ir 56 Niobium Nb 14 Cadmium Cd • Iron Fe 57 Nitrogen N 15 Calcium Ca • Krypton Kr 58 Osmium Os 17 Carbon C 59 Oxygen O 18 Cerium Ce 60 Palladium Pd 19 Cesium Cs 61 Phosphorus P 20 Chlorine Cl 62 Platinum Pt 21 Chromium Cr 22 Cobalt Co 23 Copper Cu Francium Hg return Prefixes mono- 1 di2 tri3 tetra- 4 penta- 5 hexa- 6 heptaoctanonadecaundecadodeca- 7 8 9 10 11 12 return