Stoichiometry: Calculations with Chemical Formulas and Equations
advertisement

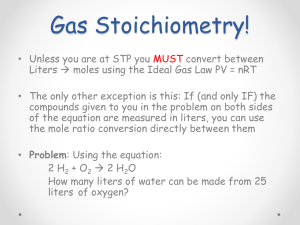
Introduction: Matter and Measurement Stoichiometry Units of Measurement Stoichiometry SI Units • Système International d’Unités • Uses a different base unit for each quantity Stoichiometry Metric System Prefixes convert the base units into units that are appropriate for the item being measured. Stoichiometry THE METRIC SYSTEM Stoichiometry WHY DO WE USE THE METRIC SYSTEM? • Almost all other countries are using the metric system • Other countries’ companies are refusing to buy products from the U.S. if not labeled in metric units • Scientists need a universal way to communicate data (SI Units) Stoichiometry APPROXIMATE CONVERSIONS BETWEEN METRIC & US LENGTH UNITS • A meter is about the same length as a yard • A meter is about three feet long • A decimeter is about four inches long • An inch is about 25 millimeters • A foot contains about 30 centimeters • A foot contains about 3 decimeters Stoichiometry WHAT DOES THE METRIC SYSTEM MEASURE? • The gram measures mass or how much something weighs • The liter measures volume which is used when measuring liquids • The meter measures the length of an object or the distance from place to place Stoichiometry THE METRIC CONVERSION CHART (STAIRCASE METHOD) Kilo 1000 Hecto units 100 Deka units 10 To convert to a smaller unit, move decimal point to the right or multiply. Basic Unit Deci 0.1 Centi units 0.01 Milli units 0.001 units To convert to a larger unit, move decimal point to the left or divide units Stoichiometry TRY THIS USING THE STAIRCASE METHOD 1000 mg = ______ g Step 1: Determine if you are going to go up or down the ladder. Step 2: Determine how many steps there are from milligrams to grams. Step 3: Move the decimal point the amount of places that was determined in steps 1 & 2. Stoichiometry TRY THIS USING THE STAIRCASE METHOD 1000 mg = ______ 1 g Step 1: Determine if you are going to go up or down the ladder. Step 2: Determine how many steps there are from milligrams to grams. Step 3: Move the decimal point the amount of places that was determined in steps 1 & 2. Stoichiometry TRY THIS USING THE STAIRCASE METHOD .15 L = __________ ml Stoichiometry TRY THIS USING THE STAIRCASE METHOD .15 L = __________ ml 150 Stoichiometry Volume • The most commonly used metric units for volume are the liter (L) and the milliliter (mL). □ A liter is a cube 1 dm long on each side. □ A milliliter is a cube 1 cm long on each side. Stoichiometry Uncertainty in Measurements Different measuring devices have different uses and different degrees of accuracy. Stoichiometry Temperature: A measure of the average kinetic energy of the particles in a sample. Stoichiometry Temperature • In scientific measurements, the Celsius and Kelvin scales are most often used. • The Celsius scale is based on the properties of water. □ 0C is the freezing point of water. □ 100C is the boiling point of water. Stoichiometry Temperature • The Kelvin is the SI unit of temperature. • It is based on the properties of gases. • There are no negative Kelvin temperatures. • K = C + 273.15 Stoichiometry Temperature • The Fahrenheit scale is not used in scientific measurements. • F = 9/5(C) + 32 • C = 5/9(F − 32) Stoichiometry A Standard Measurement System The Metric System Stoichiometry When and why was the metric system invented? • The metric system was invented in 1790 • The metric system was invented because countries were using many different systems of measurement causing confusion and lack of consistency Stoichiometry Who invented the metric system? • The metric system was invented by a group of French scientists Stoichiometry Metric System • A system of measurement used by the majority of countries on Earth based on the number 10 Stoichiometry A Standard Measurement System The International System of Units (SI) Stoichiometry Scientists all over the world use the International System of Units to measure: • • • • • • Length Volume Mass Density Temperature Time Stoichiometry Figure 1: Calculating - How much larger is a kilo- than a deka-? • 100 times Stoichiometry Reading Checkpoint (page 45): SI units are based on multiples of what number? • SI units are based on multiples of 10 • Add a zero • Subtract a zero Stoichiometry Key Concept: Why do scientists use a standard measurement system? • Using SI as the standard system of measurement allows scientists to compare data and communicate with each other about their results • Using SI measurement also allows experiments to be repeated and most importantly achieve a desired resultStoichiometry Length Stoichiometry What is length? • Length is the distance from one point to another Stoichiometry Length Units of Length Stoichiometry The basic unit of length in the SI system is the … • The basic unit of length in the SI system is the meter Stoichiometry The two units that measure the length of smaller objects are, … • millimeter • centimeter Stoichiometry Complete the Table Below Prefix Meaning Unit of Length milli one-hundredth meter one-thousand Stoichiometry Complete the Table Below Prefix Meaning milli One-thousandth Unit of Length one-hundredth meter one-thousand Stoichiometry Complete the Table Below Prefix Meaning Unit of Length milli One-thousandth millimeter one-hundredth meter one-thousand Stoichiometry Complete the Table Below Prefix Meaning Unit of Length milli One-thousandth millimeter centi one-hundredth meter one-thousand Stoichiometry Complete the Table Below Prefix Meaning Unit of Length milli One-thousandth millimeter centi one-hundredth centimeter meter one-thousand Stoichiometry Complete the Table Below Prefix Meaning Unit of Length milli One-thousandth millimeter centi one-hundredth centimeter none meter one-thousand Stoichiometry Complete the Table Below Prefix Meaning Unit of Length milli One-thousandth millimeter centi one-hundredth centimeter none one meter one-thousand Stoichiometry Complete the Table Below Prefix Meaning Unit of Length milli One-thousandth millimeter centi one-hundredth centimeter none one meter kilo one-thousand Stoichiometry Complete the Table Below Prefix Meaning Unit of Length milli One-thousandth millimeter centi one-hundredth centimeter none one meter kilo one-thousand kilometer Stoichiometry Length Measuring Length Stoichiometry The longer lines on the metric ruler are called… • centimeters Stoichiometry The shorter lines on the metric ruler are called… • millimeters Stoichiometry Checkpoint One centimeter is divided into how many millimeters? • 10 millimeters (mm) Stoichiometry Figure 2: Calculating: Measure the turtle in figure 2 from the rear of its shell to the tip of its nose. Record its length in both centimeters and millimeters. • 10.5 cm • 105 mm Stoichiometry Density Stoichiometry Density • The measure of how much mass is contained in a given volume Stoichiometry The formula of density is: Density = Mass / Volume Stoichiometry Figure 5: Comparing Densities - Inferring: Which item has the greater density? • The bowling ball • Since the bowling bowl has a greater mass, it has a greater density, even though both balls have the same volume Stoichiometry Density Units of Density Stoichiometry Why is density expressed as a combination of two different units? • Because density is actually made up of two other measurements – mass and volume – an objects density is expressed as a combination of two units Stoichiometry Two Common Units For Density • Grams per cubic centimeter (g/cm³) • Grams per milliliter (g/mL) Stoichiometry Math Practice: What is the density of a wood block with a volume of 125 cm³ and a mass of 57 g? Density = mass / volume Density = 57 g / 125 cm³ Density = 0.46 g/ cm³ Stoichiometry Math Practice: What is the density of a liquid with a mass of 45 g and a volume of 48 mL? Density = mass / volume Density = 45 g / 48 mL Density = 0.94 g/mL Stoichiometry Density Densities of Common Substances Stoichiometry The density of a substance is the ______for all samples of that substance. • Same Stoichiometry An object will float if it is _____ _____ than a surrounding liquid. • Less dense Stoichiometry Figure 6: Applying Concepts: How could you use density to determine whether a bar of metal is pure gold? • If the bar of gold has a density that is greater than or less than 19.3 g/cm³, then the sample is not pure gold. Densities of Some Common Substances Substance Density (g/cm³) Air 0.001 Ice 0.9 Water 1.0 Aluminum 2.7 Stoichiometry Gold 19.3 Checkpoint Will an object with a density of 0.7 g/cm³ float or sink in water? • An object that has a density of 0.7 g/cm³ will float in water (1 g/cm³) because it is less dense than water Stoichiometry Density: Physical property of a substance m d= V Stoichiometry Time Stoichiometry Time Units of Time Stoichiometry What is the SI unit used to measure time? • The second(s) is the SI unit to measure time Stoichiometry Common Conversions for Time 1s = = 1h 60 s = Stoichiometry Common Conversions for Time 1s 1h = 1,000 ms = 60 s = Stoichiometry Common Conversions for Time 1s = 1,000 ms 1 min = 60 s 1h = Stoichiometry Common Conversions for Time 1s = 1,000 ms 1 min = 60 s 1h = 60 min Stoichiometry Time Measuring Time Stoichiometry Why would a stop watch be used to measure time in an important race? • Because stop watches measure in units smaller than the second • These measurements include the tenth and hundredth of a second Stoichiometry Checkpoint - How many milliseconds are in one second? • 1,000 milliseconds Stoichiometry Temperature Stoichiometry Temperature Units of Temperature Stoichiometry A common unit to measure temperature is the ___ ___. • Celsius scale Stoichiometry Water freezes at ______ and boils at ______. • 0 °C • 100 °C Stoichiometry The normal human body temperature is approximately ________. • 37 °C Stoichiometry What is the official SI unit for temperature? • The Kelvin Scale (°K) • 0 °K = -273 °C Stoichiometry Figure 8: Measuring Temperature - Observing: At what temperature on the Kelvin scale does water boil? • 373 °K Stoichiometry What is absolute zero? • Absolute zero is considered by scientists to be the coldest temperature possible • 0 °K or –273 °C Stoichiometry Temperature Measuring Temperature Stoichiometry What instrument is used to measure temperature? • Thermometer Stoichiometry Volume Stoichiometry Volume • The amount of space an object takes up Stoichiometry Volume Volume of Liquids Stoichiometry When measuring the volume of a liquid, scientists use a unit known as the… • Liter (L). Stoichiometry To measure the volume of smaller liquids, the _________ is used. • Milliliter (mL) Stoichiometry The instrument used to measure the volume of liquids is called the… • Graduated cylinder. Stoichiometry This instrument has markings that are in increments of… • 1 milliliter (mL) Stoichiometry Meniscus • The curve in the top surface of water in the graduated cylinder Stoichiometry Figure 4: Observing - What is the proper way to read a meniscus? • Read the milliliter marking at the bottom of the curve Stoichiometry Volume Volume of Rectangular Solids Stoichiometry Common Conversions For Volume 1L = = 1 mL 1,000 cm³ = Stoichiometry Common Conversions For Volume 1L 1 mL = 1,000 mL = 1,000 cm³ = Stoichiometry Common Conversions For Volume 1L = 1,000 mL 1 L = 1,000 cm³ 1 mL = Stoichiometry Common Conversions For Volume 1L = 1,000 mL 1 L = 1,000 cm³ 1 mL = 1 cm³ Stoichiometry How can the volume of a solid object such as a shoebox be measured? • To measure a solid objects that are regular shaped, a formula for volume can be applied • To measure a rectangular object such as a shoebox, multiply the object’s Stoichiometry length, width, and height The SI unit known for measuring solids with a larger volume is known as the… • Cubic meter (m³). Stoichiometry The formula for calculating the volume of a rectangular object is: Volume = Length x Width x Height Stoichiometry Why is the unit cm³ used when calculating the volume of a rectangular object? • When multiplying the object’s length, width and height, the cm units are also multiplied cm x cm x cm = cm³ Stoichiometry Suppose a cereal box is 10 centimeters long, 4 centimeters wide, and 20 centimeters high. What would be the volume of the box? Volume = Length x Width x Height Volume = 10 cm x 4 cm x 20 cm Volume = 800 cm³ Stoichiometry Checkpoint What is a cubic meter? • The SI unit used to measure solids with a larger volume • A cubic meter is equal to the volume of a cube that measures 1 meter on each side Stoichiometry Volume Volume of Irregular Solids Stoichiometry How is the volume of an irregular solid such as a rock measured? • To measure the volume of an irregular solid, immerse the object in water, and measure how much the water level rises • Water displacement method Stoichiometry How does the water displacement method work? • Record the volume of water in the graduated cylinder • Carefully place the irregular solid into the water. Record the volume of the water plus the object • Subtract the volume of the water alone from the volume of the water plus the object Stoichiometry Uncertainty in Measurement Stoichiometry Significant Figures • The term significant figures refers to digits that were measured. • When rounding calculated numbers, we pay attention to significant figures so we do not overstate the accuracy of our answers. Stoichiometry Significant Figures 1. All nonzero digits are significant. 2. Zeroes between two significant figures are themselves significant. 3. Zeroes at the beginning of a number are never significant. 4. Zeroes at the end of a number are significant if a decimal point is written in the number. Stoichiometry Significant Figures • When addition or subtraction is performed, answers are rounded to the least significant decimal place. • When multiplication or division is performed, answers are rounded to the number of digits that corresponds to the least number of significant figures in any of the numbers used in the calculation. Stoichiometry Relating Significant Figures to the Uncertainty of a Measurement What difference exists between the measured values 4.0 g and 4.00 g? Solution Many people would say there is no difference, but a scientist would note the difference in the number of significant figures in the two measurements. The value 4.0 has two significant figures, while 4.00 has three. This difference implies that the first measurement has more uncertainty. A mass of 4.0 g indicates that the uncertainty is in the first decimal place of the measurement. Thus, the mass might be anything between 3.9 and 4.1 g, which we can represent as 4.0 ± 0.1 g. A measurement of 4.00 g implies that the uncertainty is in the second decimal place. Thus, the mass might be anything between 3.99 and 4.01 g, which we can represent as 4.00 ± 0.01 g. Without further information, we cannot be sure whether the difference in uncertainties of the two measurements reflects the precision or accuracy of the measurement. Stoichiometry PRACTICE EXERCISE A balance has a precision of ± 0.001 g. A sample that has a mass of about 25 g is placed on this balance. How many significant figures should be reported for this measurement? Answer: five, as in the measurement 24.995 g Stoichiometry SAMPLE EXERCISE 1.6 Determining the Number of Significant Figures in a Measurement How many significant figures are in each of the following numbers (assume that each number is a measured quantity): (a) 4.003, Four; the zeros are significant figures (b) 6.023 1023, Four; the exponential term does not add to the number of significant figures. (c) 5000? One. We assume that the zeros are not significant when there is no decimal point shown. If the number has more significant figures, a decimal point should be employed or the number written in exponential notation. Thus, 5000. has four significant Stoichiometry figures, whereas 5.00 103 has three. PRACTICE EXERCISE How many significant figures are in each of the following measurements: Answers: (a) 3.549 g, (a) four, (b) two, (b) 23 104 cm, (c) three (c) 0.00134 m3? Stoichiometry Determining the Number of Significant Figures in a Calculated Quantity The width, length, and height of a small box are 15.5 cm, 27.3 cm, and 5.4 cm, respectively. Calculate the volume of the box, using the correct number of significant figures in your answer. Solution The volume of a box is determined by the product of its width, length, and height. In reporting the product, we can show only as many significant figures as given in the dimension with the fewest significant figures, that for the height (two significant figures): When we use a calculator to do this calculation, the display shows 2285.01, which we must round off to two significant figures. Because the resulting number is 2300, it is best reported in exponential notation, 2.3 103, to clearly Stoichiometry indicate two significant figures. PRACTICE EXERCISE It takes 10.5 s for a sprinter to run 100.00 m. Calculate the average speed of the sprinter in meters per second, and express the result to the correct number of significant figures. Answer: 9.52 m/s (3 significant figures) Stoichiometry Determining the Number of Significant Figures in a Calculated Quantity A gas at 25°C fills a container whose volume is 1.05 103 cm3. The container plus gas have a mass of 837.6 g. The container, when emptied of all gas, has a mass of 836.2 g. What is the density of the gas at 25°C? Solution To calculate the density, we must know both the mass and the volume of the gas. The mass of the gas is just the difference in the masses of the full and empty container: (837.6 – 836.2) g = 1.4 g In subtracting numbers, we determine the number of significant figures in our result by counting decimal places in each quantity. In this case each quantity has one decimal place. Thus, the mass of the gas, 1.4 g, has one decimal place. Using the volume given in the question, 1.05 103 cm3, and the definition of density, we have In dividing numbers, we determine the number of significant figures in our result by counting the number of significant figures in each quantity. There are two significant figures in our answer, corresponding to the smaller number of significant figures in the two numbers that form the ratio. Stoichiometry To how many significant figures should the mass of the container be measured (with and without the gas) in Sample Exercise 1.8 in order for the density to be calculated to three significant figures? Answer: five (In order for the difference in the two masses to have three significant figures, there must be two decimal places in the masses of the filled and empty containers.) Stoichiometry Accuracy versus Precision • Accuracy refers to the proximity of a measurement to the true value of a quantity. • Precision refers to the proximity of several measurements to each other. Stoichiometry