Source Documentation, CRFs, and Electronic Records

Diane Glaviano
Kathy Gilmartin
What are they?
Why is it so important?
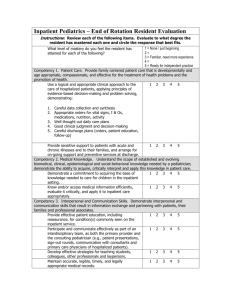
Consistency of source documentation for specific data points
- Progress Records
- Vital Sign Worksheets
- MRI/CT Scan/PET scan reports
- Pathology Reports
- Drug Infusion Sheets
Fundamentals
- Organization!! colored labels, highlighting data points, chronological order, sections,
Principle Investigator over site, meeting regularly with PI for chart review and signature of agreement of data confirmation.
Inclusion / Exclusion Criteria
Ensure that all source documentation is current and imaging, assessments and labs all fall within the proper study time windows.
If the participant does not meet all inclusion/exclusion criteria, you will have to seek a waiver from the IRB or Compassionate
Use request from the FDA.
Show all calculations if required.
SC and PI to sign off on the inclusion/exclusion worksheet.
All Scripts (Office Visits)
Physician notes
Clinical Summary
Medication List
Scans, Labs, EKG/ECHO
Jeff Charts (inpatient records)
Inpatient records
Inpatient scans/labs/notes
Inpatient medication lists
On Care (infusion center)
Treatment summaries
Nursing notes
Infusion/treatment instructions and notes
Mosaiq (Radiation)
Radiation treatment plan/summary
Dictations
JeffNotes Signature Plus
Inpatient records
EPIC will be coming in 2017
Response Evaluation Criteria In Solid Tumors
(RECIST) is a set of published rules that define when tumors in cancer patients improve
("respond"), stay the same ("stabilize"), or worsen ("progress") during treatment.
Patient is added to system once registered in JeffTrial
Request all RECIST readings as soon as possible (may take up to 2 weeks)
Clinical Research Data base
Enter off Treatment, off study and death dates
Enter follow-up data (including last and next
FU date) https://jefftrial.tjh.tju.edu/login/
Oracle
Medidata
Inform
REDCap (excel can be imported)
Do not share passwords
Enter data at a time of the day when you are most alert; avoid interruptions.
Data can always be added the next day to ensure accuracy .
Ensure that when sending any documents outside of TJU (ie. Sponsor) that they are properly de-identified with the study ID only
Sharpie / PDF program that de-identifies information: Medical De-identification System
(MeDS),
External Contractors – At the time of signing their contacts for study initiation, there is a clause in the contract that covers all aspects of HIPPA for outside auditors.
The Data and Safety Monitoring Plan (DSMP) of
Thomas Jefferson University’s Sidney Kimmel
Cancer Center (SKCC) provides for the oversight and monitoring of ongoing clinical trials to ensure the safety of participants and the validity and integrity of the data of these trials.
The DSMP shall also ensure the appropriate termination of studies for which significant benefits or risks have been identified or if it has been discovered that the trial cannot be concluded successfully.
The DSMP requires that every Sidney Kimmel
Cancer Center protocol include a data and safety monitoring plan.
Data and Safety Monitoring Committee (DSMC) is the Data and Safety Monitoring Board (DSMB) for the SKCC. The DSMC is a multidisciplinary committee charged with overseeing the monitoring of safety of participants in clinical trials, and the conduct, progress, validity, and integrity of the data for all clinical trials at the
Thomas Jefferson University SKCC.
The committee meets quarterly to review the progress and safety of all active research protocols that are not monitored by another data and safety monitoring committee or board.
Oversee data collection
Review source documentation and case report forms
Ensure regulatory compliance
Resolve data queries
Conduct interim analyses as requested by clients
For Investigator Initiated Trials – 2-3X/year depending on enrollment
“Snap Shot” of the overall conduct of the study
- Consenting process and with accuracy of signatures, dates, documentation of the consenting process with study team properly identified on the DOAL.
- Review of Regulatory Binder, includes initial
IRB submission, AMDs, consents, Monitoring Log,
DOAL, Training Log, Study team correspondence,
SKCC – Internal / External
Audit at 10% and 50% accrual and later close out of the study
Developed in response to the soaring costs associated with managing the distribution, storage, and retrieval of records used in conjunction with the FDA.
Security concerns surrounding wet ink signatures.
Part 11 in particular applies to records in electronic form that are created, modified, maintained, archived, retrieved, or transmitted under any records requirements set forth in
Agency regulations.
Maintains Integrity and Proving Authenticity of the record.